As suggested from recent findings, the role of alcohol in HNC seems to be broader than that of a simple risk factor. In this entry, authorsarrative review we report evidence from past studies to clarify the role of alcohol consumption in head and neck cancer (HNC) onset. Moreover, we further explore the role of oral microbiota, oxidative stress and genetic expression alterations due to alcohol drinking. Although alcohol is not the exclusive risk factor for HNC carcinogenesis, it plays a major role in the etiopathogenesis of both primary tumors and their recurrences, especially by means of ethanol and its metabolic products. Alcohol modifies oral microbiota, enhances intracellular oxidative stress, expose epithelial cells to carcinogens and alters cellular genetic expressions by promoting epigenetic mutations, DNA damage, and inaccurate DNA repair related to the formation of DNA adducts.
The relationship between alcohol and HNC has been well established but, unfortunately, there is no clear threshold effect of alcohol for oncogenic patients, so that prevention and monitoring with long-term markers of alcohol consumption (especially those detected in the hair) that relay information on the actual alcohol drinking habits, seem to be the most effective ways to contrast its prevalence (and complications) in HNC drinker-patients. These conclusions seem to be especially important nowadays since, despite the established association between alcohol and HNC, a concerning pattern of alcohol consumption misconducts has been found in both in the general population and HNC survivors. Interestingly, evidence that we report on HNC etiopathogenesis suggests a key role of polyphenols and alkylating agents for patient management, especially in case of heavy chronic drinkers.
1. Introduction
Worldwide, head and neck cancer (HNC) accounts for more than 890,000 cases and 450,000 deaths annually
[1]. Head and neck cancer is a malignancy, associated with the advanced stage at presentation and heavy outcomes (mean 5-year survival <50%), that occurs more often in the lips and oral cavity, nasopharynx, oropharynx, hypopharynx, and larynx; squamous cell carcinoma (SCC) represents the prevalent histology
[2][3][2,3].
Alcohol abuse may result in significant mental
[4][5][6][7][8][4,5,6,7,8] or physical health problems
[9][10][11][12][9,10,11,12]. Furthermore, when consumed during gestation, it may induce severe damage to the newborns
[13][14][15][16][17][18][19][20][13,14,15,16,17,18,19,20]. Alcohol is a well-known carcinogen compound but it is still underestimated in the general population, partially also because of the alcohol industry’s extensive misrepresentation of evidence about the alcohol-related risk of cancer
[21][22][21,22]. Alcohol drinking, together with tobacco smoking, and human papillomavirus (HPV) infection (
Table 1) are HNC-recognized risk factors
[23][24][25][26][23,24,25,26]. Interestingly, the role of alcohol in HNC seems to be broader than that of a simple risk factor, as suggested from recent findings which highlighted how significant inverse association exists between alcohol drinking and prognosis among HNC patients
[27][28][27,28]. It has been reported that, in 2012, a total of 203,511 cases of the oral cavity, oropharyngeal, hypopharyngeal, and larynx cancer were attributable to alcohol consumption (179,559 men and 23,952 women)
[29]. The proportion of HNC cases attributable to alcohol is still increasing, emphasizing the importance of alcohol consumption limitation to prevent HNC. Alcohol use among HNC survivors negatively impacts patient outcomes and is an important risk factor for recurrent and second primary tumors. Despite recommendations from several cancer societies, alcohol consumption remains a common problem in this population.
[30]. The estimate of the real alcohol consumption (based not only on what the patient declared during the anamnesis) would be of support in consolidating the correlation with the onset of HNC.
Table 1. Major differences between HPV + and HPV - HNSCC (mainly related to alcohol abuse and smoke). Alcohol is a major determinant of aggressive HNCs. HNSCC, head and neck squamous cell carcinomas; HPV, human papillomavirus.
| |
HPV + HNSCC |
HPV - HNSCC |
| Main risk factors |
Sexual contact, HPV type 16 and 18 |
Alcohol and smoking |
| Tumor site |
Oropharynx |
Non-oropharyngeal sites |
| Histopathology |
Basaloid, non-keratinizing, poorly differentiated |
Keratinizing, moderately differentiated |
| Main carcinogenic factor |
Viral protein E6 and E7 action |
DNA damage and inaccurate DNA repair promoted by alcohol catabolism and smoke carcinogen components action |
| Responsiveness to chemoradiation |
Better than HPV - HNSCC |
Worse than HPV + HNSCC |
| Prognosis |
Better than HPV - HNSCC |
Worse than HPV + HNSCC |
| Prevention |
HPV vaccine, condom |
Alcohol and smoking abstinence |
2. Head and Neck Cancer and Alcohol
2.1. Diagnosis and Treatments
The HNC diagnosis usually includes laryngoscopy, imaging [Positron emission tomography/X-ray computed tomography (PET/CT) and magnetic resonance imaging (MRI)], and biopsy of the primary lesion
[31][32][33][34][35][31,32,33,34,35]. As technology progresses, the development of non-invasive diagnostic tools in the field of head and neck oncology has been examined; the molecular analysis of tumor’s genetic features based on circulating malignance derivatives, such as circulating tumor DNA, intact circulating tumor cells (CTCs), and exosomes in patients’ blood, namely liquid biopsy, has become a concrete possible approach to improve diagnostics, treatment planning, and post-treatment surveillance in patients with the potentially curable disease
[36][37][38][39][36,37,38,39].
Treatment possibilities include tumor resection (primary and/or secondary tumor), radical neck dissection, immunotherapy, radiotherapy, checkpoint inhibitors (mainly targeting the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)), programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), and chemotherapy
[40][41][42][43][44][45][40,41,42,43,44,45]. Recently, it has been showing how, in locally advanced HNSCC, the CTCs and the circulating tumor microemboli (CTM) have a significant prognostic impact on the potential role as predictors of induction chemotherapy benefit
[46].
It is believed that the majority of oral cancers develop from oral potentially malignant lesions (OPMLs)
[47]. Though they can be easily detected during screening, risk stratification is difficult. During screening, clinicians often find it difficult to distinguish OPMLs from benign lesions, and predicting OPMLs at risk of malignant transformation could be particularly challenging
[47]. DNA aneuploidy has been known to be a marker of malignancy in a number of sites, including the oral cavity
[47]. Indeed, DNA ploidy and chromatin organization of cells collected from OPMLs can identify lesions at high risk of progression several years prior
[48]. This non-invasive test would enable clinicians to triage high-risk OPMLs for closer follow-up, while low-risk lesions can undergo less frequent biopsies, reducing the burden on healthcare resources
[48]. Quite interestingly, in a study on individuals with Fanconi anemia (people with a 500-fold to 700-fold elevated risk, much earlier onset, and limited therapeutic options for oral SCC compared with the general population), a careful inspection of the oral cavity associated with brush biopsy-based cytology could identify visible oral lesions, either malignant or potentially malignant, that warrant treatment
[49].
2.2. Alkylating Agents
Because of the mentioned key role of genetic and epigenetic alterations in HNC, treatment protocols still include the use of alkylating agents (AAs). AAs are a heterogeneous class of drugs that interfere with the cell’s DNA and inhibit cancer cells’ growth, playing a major role in HNC
[50]. These genotoxic agents modify the DNA by adding binding an alkyl group to the guanine base of DNA at the number 7 nitrogen atom of the purine ring, either directly or after metabolic conversion to reactive intermediates
[51][52][51,52]. These drugs produce numerous side effects targeting many organs and apparats, such as the gastrointestinal tract, bone marrow, testicles, and ovaries; furthermore, most of the alkylating agents are also carcinogenic
[53][54][53,54]. AAs still play a major role in the chemotherapeutic treatment of HNC, especially cisplatin and methotrexate, in recurrent metastatic cancer, but the focus is gradually shifting to non-conventional systemic chemotherapy, especially targeted therapy and immunotherapy, which affect the tumor microenvironment and have a potentially favorable impact on HNC management
[55][56][57][55,56,57].
2.3. Alcohol Abuse Detection
Despite the numerous proposed biomarkers in many studies, no laboratory test is sufficiently reliable alone to support a diagnosis of alcohol use disorder (AUD)
[58][59][58,59]. Sensitivity and specificity should be high for alcohol abuse biomarkers, but in reality, they mostly fluctuate considerably and depend on the involved population. Furthermore, the ideal markers should reflect an individual’s consumption of alcohol, both chronically (screening markers) and acutely (relapse markers), and, from this, the given title of “state” markers (in contrast to the “trait” markers that predict the predisposition to develop alcoholism)
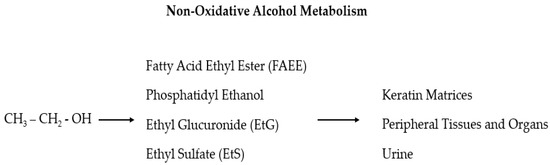
[60][61][60,61]. The use of long-term diagnostic tools gives crucial information on the real alcohol consumption of HNC patients so that a series of recently found useful biomarkers, which can be detected in the hair, is now in the spotlight: ethyl glucuronide (EtG), fatty acid ethyl esters (products of non-oxidative ethanol metabolism), phosphatidyl ethanol, acetaldehyde adducts to protein, and 5-hydroxytryptophol
[18][62][63][64][18,62,63,64]. The main advantages of this sample material are that compounds with a relatively short lifetime in blood, but with a strong correlation to alcohol consumption, can be entrapped in the hair and are detectable for a longer time (also for years depending on the length of the hair) and at a relatively high concentration
[64][65][64,65]. In particular, EtG and ethyl sulfate (EtS) are two non-oxidative ethanol metabolites (
Figure 1) secreted by the liver which are mainly used as markers of alcohol intake related to incidents
[66][67][68][69][70][71][66,67,68,69,70,71]. These two markers for recent alcohol intake can be detected in the blood for approximately 10 h after a small to moderate alcohol intake and up to 5 days after large and repeated alcohol intakes
[67][68][69][67,68,69]. As the efficacy of these two tests has been demonstrated in multiple settings, it has been also suggested that EtG and EtS should be included in screening tests for injured or at-risk for alcohol abuse people (including pregnant women) to investigate the possible association between residual alcohol effects and injuries, and to verify alcohol abstinence in cases of substance-related disorders
[72][73][72,73].
Figure 1. In the liver, ethanol is metabolized via oxidative and non-oxidative (less than 1%) ways. In the non-oxidative pathway, alcohol is finally processed as fatty acid ethyl ester (FAEE), phosphatidyl ethanol, ethyl glucuronide (EtG), and ethyl sulfate (EtS).