Proper formation of the skeleton during development is crucial for the mobility of humans and the maintenance of essential organs. The production of bone is regulated by osteoblasts and osteoclasts. An imbalance of these cells can lead to a decrease in bone mineral density, which leads to fractures. The data demonstrated that isolated cells can be differentiated into functional osteoclasts, as indicated by the 92% and 83% of cells that stained positive for TRAP and Cathepsin K, respectively. Furthermore, isolated cells remain viable and terminally differentiate into osteoclasts when stimulated with RANKL. These data demonstrate that cells isolated from human femoral heads can be differentiated into osteoclasts to study bone disorders during development and adulthood.
1. Introduction
The Formation of the skeleton during embryogenesis and early stages of development is crucial for proper protection, support, and function of human organ systems
[1][2][3][4][1,2,3,4]. This process is governed by two major bone cell types, which include osteoblasts and osteoclasts
[5][6][7][8][9][10][5,6,7,8,9,10]. Osteoblasts are mononucleated cells responsible for forming new bone and derive from mesenchymal stem cell (MSC) progenitors
[11][12][13][14][11,12,13,14]. Contrarily, osteoclasts are multinucleated immune cells that derive from hematopoietic stem cells (HSCs) and resorb old or damaged bone
[8][10][15][16][17][18][8,10,15,16,17,18]. The crosstalk between these two cell types is critical for the formation of early structures, such as digits, and bone homeostasis throughout adulthood
[1][19][20][1,19,20]. Indeed, about 10% of the adult human skeleton is renewed each year and is dependent on the balance between osteoblasts and osteoclasts
[5][8][9][21][5,8,9,21]. However, as humans age, the balance shifts toward bone resorption, and may lead to bone disorders such as osteopenia and osteoporosis (OP)
[21][22][23][24][25][26][21,22,23,24,25,26].
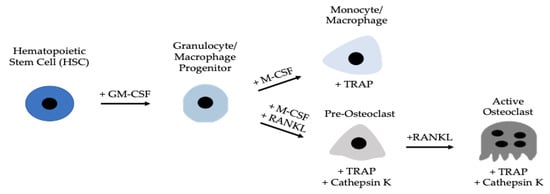
Osteoclastogenesis and the activity of osteoclasts are reliant on osteoblasts
[16][27][28][29][30][16,27,28,29,30]. Osteoblasts secrete two proteins that are necessary for osteoclast function and differentiation: macrophage colony stimulating factor (M-CSF) and receptor activator of nuclear factor kappa-β ligand (RANKL)
[31][32][33][31,32,33]. As osteoblasts secrete M-CSF, this ligand subsequently binds to colony-stimulating factor-1 (c-fms) receptors located on granulocyte/macrophage progenitors (GMPs) or peripheral blood mononuclear cells (PBMCs)
[34][35][36][34,35,36]. Here, GMPs differentiate into monocyte/macrophage cells, which are considered osteoclast precursors
[37]. Simultaneously, osteoblasts are secreting RANKL, which will bind to RANK receptors expressed on the cell surfaces of monocytes/macrophages
[38][39][38,39]. The monocytes/macrophages can then terminally differentiate into functional osteoclasts, which will be responsible for resorbing bone (
Figure 1). The M-CSF and RANKL signaling pathways promote cell differentiation, survival, and proliferation by activating the Akt, Erk, NF-κB, and MAPK pathways
[18].
Figure 1. Development of an active osteoclast. Hematopoietic stem cells (HSCs) differentiate into GMPs, which express c-fms receptors that M-CSF ligands can bind. After binding, GMPs differentiate into monocyte/macrophage precursors, that further differentiate into pre-osteoclasts and active osteoclasts when RANKL binds to RANK receptors. Active osteoclasts are multinucleated and express TRAP and Cathepsin K.
Osteoclasts are expressed predominantly during bone resorption, making them difficult to isolate in high concentrations so as to study osteoclastogenesis
[40][41][42][40,41,42]. The isolation process becomes more difficult when obtaining osteoclasts or pre-osteoclasts directly from the bone marrow of patients with bone disorders, such as OP or osteoarthritis (OA). Moreover, previous studies suggest that while osteoclasts may be isolated, they are obtained at low concentrations, especially when terminally differentiated
[42][43][44][45][42,43,44,45]. Furthermore, as these cells do not proliferate, it is difficult to maintain and keep them viable
[43][44][45][43,44,45]. These cells also undergo apoptosis rapidly when isolated, posing many challenges when maintaining them
[46]. To combat these challenges, previous studies have utilized PBMCs obtained from human blood
[32][36][37][41][47][32,36,37,41,47]. As these cells are consisted of monocytes/macrophages, this method is a powerful technique to study osteoclastogenesis. However, less data are available regarding cells isolated directly from the bone marrow of humans, which may provide additional insight into the function of osteoclasts in the bone microenvironment.
2. RAW264.7 Cells Do Not Differentiate Readily into Osteoclasts
RAW264.7 cells are murine monocytes/macrophages that can be differentiated into osteoclasts. However, the extent of osteoclastogenesis is not clear, and this cell line may be utilized to observe osteoclast activity
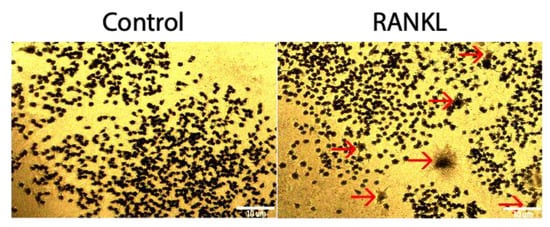
[16]. To determine if RAW264.7cells could be differentiated into osteoclasts using 10 ng/mL of RANKL, a TRAP assay was utilized. Compared to the control group that was not stimulated with RANKL, the RANKL stimulated group was not significantly higher, as only 2.5% of cells stained positive for TRAP (
Figure 23).
Figure 23. TRAP assay of RAW264.7 cells stimulated with RANKL. After 5 days of treatment with 10 ng/mL of RANKL, 2.5% of stimulated cells stained positive for TRAP, whereas 0.09% of control cells not treated with RANKL stained positive for TRAP. Red arrows designate a TRAP positive cell. Images were acquired with a 10× objective lens and scale bars are set to 10 μm. All of the experiments were conducted in triplicate.
3. Cells isolated from Female and Male OA Patients Differentiate into Functional Osteoclasts
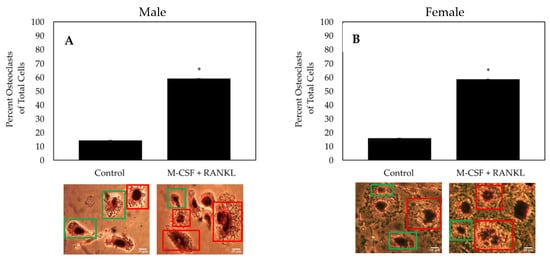
As both osteoclasts and macrophages can express TRAP, multinucleated cells must be identified to confirm the formation of an osteoclast. To determine the effectiveness of the differentiating cells isolated from female and male OA patients into osteoclasts, cells were treated with M-CSF and RANKL or M-CSF only (control). As indicated by TRAP positive and multinucleated cells, ~60% of cells stimulated with RANKL differentiated into osteoclasts, whereas only ~15% of control cells differentiated into osteoclasts and most remained monocytes/macrophages (
Figure 35). Furthermore, isolated cells from both male and female patients respond similarly to treatment.
Figure 35. TRAP assay of the cells isolated from the femoral heads of OA patients. (
A) Osteoclast count of male patients (
N = 5). (
B) Osteoclast count of female patients (
N = 5). After stimulating cells for 14 days with M-CSF and RANKL, or M-CSF only, osteoclasts were identified as cells that stained positive for TRAP and comprised of three or more nuclei. Representative images are displayed underneath bars. Red boxes designate TRAP positive cells with more than three nuclei and green boxes denote macrophages/monocytes. Images were acquired with a 20× objective lens and all experiments were conducted in triplicate. “*” denotes statistical significance, where
p is set to 0.05.
4. Osteoclasts Isolated from OA Patients Express TRAP and Cathepsin K
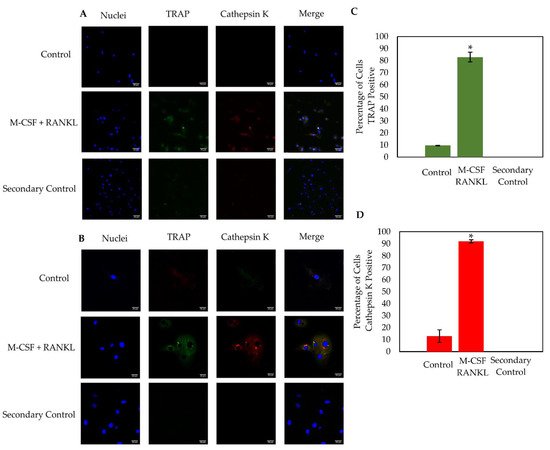
Osteoclasts express active enzymes that are responsible for degrading old or damaged bone. The most notable osteoclast markers are TRAP and Cathepsin K, as both as are highly expressed during resorption. Here, the osteoclast markers TRAP and Cathepsin K were immunostained in cells stimulated with RANKL or were left unstimulated. Cells were imaged using confocal microscopy at 20× and 63× magnification (
Figure 47A,B). Similar to the TRAP stain, the RANKL experimental group contained ~60% osteoclasts while the control group displayed ~15% osteoclasts (data not shown). Furthermore, cells stimulated with RANKL expressed high levels of both TRAP and Cathepsin K, while this expression was minimal in the control cells (
Figure 47C,D). At 63× magnification, high levels of TRAP and Cathepsin K were expressed in distinct domains within the RANKL-stimulated osteoclast, but this expression was seen very minimally in the control group, which is consistent with previous results (
Figure 47B)
[48][49][50][49,50,51].
Figure 47. Immunostaining of cells isolated from male (
N = 2) and female (
N = 1) OA patients. Confocal microscopy was utilized to image cells at 20× (
A) and 63× (
B) magnification. The population of cells stimulated with M-CSF + RANKL was ~60%, whereas the control group had ~15% osteoclasts. As displayed in the figure, M-CSF + RANKL stimulated highly expressed TRAP and Cathepsin K cells, while the control cells produced very little of these osteoclast markers (
C,
D). Cells from 10 representative images obtained at random from all three patients were counted. The RANKL stimulated cells expressed significantly higher TRAP and Cathepsin K than the control groups (
C,
D). Experiments were completed in triplicate and processed using ImageJ. All fluorescence was normalized to the secondary control. “*” denotes statistical significance, where
p is set to 0.05.
56. Findings
Proper formation of the skeleton during development is crucial for mobility and the protection of organs. The formation of new bone and the degradation of old or damaged bone is regulated by mononuclear osteoblasts and multinucleated osteoclasts [1][51][52][1,52,53]. Irregular activities of these bone cells can lead to juvenile osteoporosis in childhood, or osteopenia and osteoporosis later in adulthood [5][53][5,54]. While it is suggested that these bone disorders may arise due to hyperactivity of osteoclasts, the function of these bone resorption cells isolated directly from humans is not established [1][18][1,18]. Therefore, there is an urgent need to establish a reliable method of isolating viable bone marrow cells that can be differentiated into osteoclasts.