Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Choudhary Sobhan Shakeel and Version 2 by Rita Xu.
COVID-19 vaccines have met varying levels of acceptance and hesitancy in different parts of the world, which has implications for eliminating the COVID-19 pandemic.
- COVID-19
- vaccine acceptance
- vaccine hesitancy
- associated factors
1. Introduction
The COVID-19 pandemic has impacted many aspects of our everyday lives and changed the socio-economic fabric of the entire world [1][2][3][4][1,2,3,4]. The COVID-19 disease is caused by the highly contagious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and, at the time of its outbreak, no vaccine was available to prevent individuals from contracting the infection. Therefore, countries had to take stringent measures in order to contain the infection, including nation-wide lockdowns and border closures [5][6][7][8][5,6,7,8]. Along with countries implementing lockdowns, home-healthcare services were also optimized in order to cater to the needs of COVID-19 patients at their homes in case they either did not require hospitalization or could not be admitted to hospitals due to a lack of patient beds or other vital medical facilities [9][10][9,10]. Multi-objective home-healthcare services involving the use of artificial-intelligence models were introduced, ensuring patient availability and convenience [9]. Furthermore, home-healthcare supply-chain frameworks have also been introduced based on programming models that aid patients in selecting pharmacies, enhance the selection and routing of nurses and also help caregivers connect with patients in a timely manner [10]. Despite these protective measures, the coronavirus continued to spread and harm individuals including children, the elderly and people with medical conditions such as cancer, diabetes and respiratory diseases, who were also at the highest risk of contracting the infection [5][11][12][13][5,11,12,13]. Individuals who required access to routine medical services such as pregnant women and people with chronic conditions experienced mental health issues such as stress, depression and reduced emotional well-being [14][15][16][17][14,15,16,17]. The emotional well-being of parents and children also suffered due to the lack of educational and food resources and the enhanced stress and financial problems due to restricted and insufficient healthcare facilities, especially in less-developed countries [18][19][20][21][22][18,19,20,21,22]. Due to the overwhelming number of positive cases and the limited availability of medical devices, the pressure on healthcare workers (HCWs) has significantly increased and placed them at higher risk of contracting the contagion. Several studies have reported clinically significant depression, stress and decreased mental well-being in HCWs [23][24][23,24]. Due to these numerous harmful effects of the COVID-19 virus, there is a crucial need to develop and administer vaccines in order to eliminate this deadly pandemic [24][25][24,25].
The Coalition for Epidemic Preparedness Innovations (CEPI) has been cooperating with the World Health Organization (WHO) to aid the vaccine developers in successfully developing and deploying COVID-19 vaccines [26]. The rapid development of the COVID-19 vaccine was seen as a necessity to suppress the repeated infection waves and lower the mortality rate [27]. The COVID-19 vaccine-development efforts started in March 2020 with the first vaccine candidate entering human trials on 16 March 2020. On 4 January 2021, the United Kingdom (U.K.) became the first country to administer a COVID-19 vaccine, which was manufactured by AstraZeneca in association with Oxford University [28]. Soon, other countries started their own vaccine campaigns. For example, China administered vaccines developed by home manufacturers such as Sinopharm, Sinovac and Cansino Biologica; Russia administered its vaccine known as Sputnik V; and the United States (U.S.) has been using vaccines developed by home manufacturers including Pfizer-BioNTech, Moderna, and Johnson and Johnson [28]. The rapid and sustained administration of the COVID-19 vaccine is critical for the world to return to the pre-pandemic normalcy. As of November 2021, one hundred and seventy-five vaccines are in clinical trials, forty-one have been approved and reached the final testing phase, and seventy-five are undergoing animal trials. Although the current progress in vaccine development and administration is encouraging, social-distancing and face-mask mandates are still in place in various regions to counteract the infection [29][30][31][29,30,31]. This is because, to be effective, the COVID-19 vaccines must be administered to the majority of the world population [32]. However, variations in vaccine acceptance and hesitancy have been observed in different groups across the world [33]. This means that the world, at large, is at risk of yet another pandemic as new SARS-CoV-2 variants continue to emerge.
2. Characteristics of the Papers Included
81 papers were selected for this systematic review, with the most papers being from China (n = 12), followed by Italy (n = 8) and then the U.S (n = 8). Also included in this review were studies conducted in Saudi Arabia (n = 5), France (n = 4), Hong Kong (n = 4), Turkey (n = 4) and the U.K. (n = 4). While the majority of the studies were published in 2020, the most recent paper was published in June 2021. Six studies involved more than one country. Neumann-Böhme et al. [34][95] published a study spanning seven European countries, Lazarus et al. [35][34] focused on nineteen countries, and the research of Bono et al. [36][35] was conducted in nine countries. Taylor et al. [37][110], Salali and Uysal [38][98] and Sallam et al. [39][55] conducted their studies in two countries each. All of the studies focused on adults, with the exception of Zhang et al. [40][74], who worked with children below 18 years of age. Among the included studies, 57 surveys included the general population and 16 included HCWs. Three studies focused on multiple groups including the general population, HCWs and healthcare college students [41][42][43][68,78,88]. Two studies focused solely on dentists, dental surgeons and dental students [44][104]. Lazarus et al. [35][34] had the largest sample size (n = 13,426), while Mascarenhas et al. [44][104] had the smallest (n = 248). A total of fifty countries that reported their COVID-19 vaccine-acceptance rates are highlighted in Figure 1.
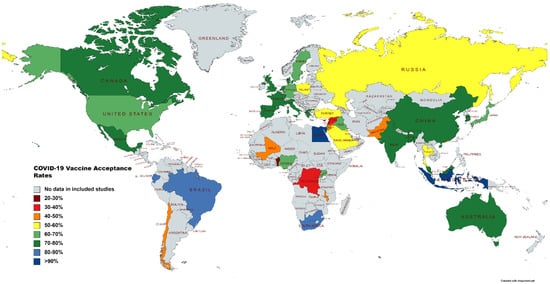

Figure 1. Map illustrating vaccine-acceptance rates worldwide.
3. Rates of COVID-19 Vaccine Acceptance
The mean COVID-19 vaccine-acceptance rates of countries are illustrated in Figure 2. Indonesia (93.30%) had the highest mean vaccine-acceptance rate followed by Egypt (90.50%), Brazil (87.15%), South Africa (85.85%), Ecuador (84.45%) and Denmark (80%). The means and standard deviations for the continents are shown in Figure 3. We found that the highest mean vaccine-acceptance rate was reported by South America (78.44%), whereas the lowest mean vaccine-acceptance rate was reported in Africa (56.59%).
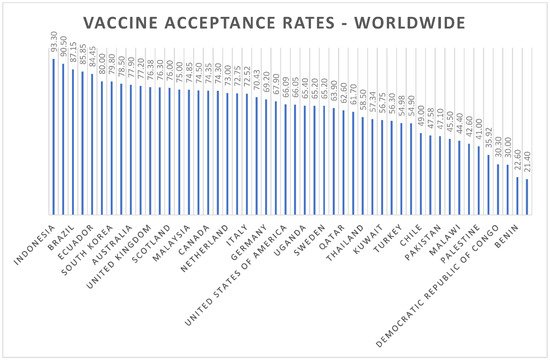
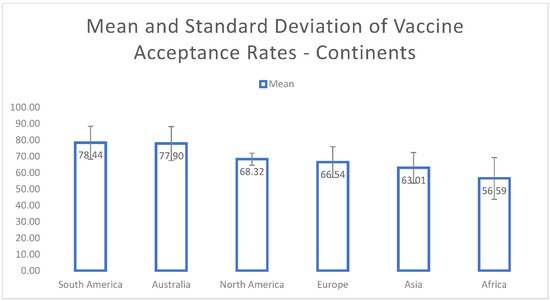

Figure 2. Worldwide COVID-19 vaccine-acceptance rates.

Figure 3. Mean and standard deviation of COVID-19 vaccine-acceptance rates for continents.
Among adults from the general population, the highest vaccine-acceptance rates were reported in Ecuador (97%), Malaysia (94.3%) and Indonesia (93.3%), and the lowest rate was reported in Lebanon (21.40%). In the healthcare workers (HCWs) category, general HCWs in China (86.20%) and nurses in Italy (91.50%) had the highest acceptance rates. The HCWs in the Democratic Republic of Congo had the lowest acceptance rate (27.70%). Among the patients with chronic diseases, those with rheumatic disease in Turkey showed a vaccine-acceptance rate of 29.2%, adolescent cancer survivors in the U.S. had an acceptance rate of 63%, and patients with type-two diabetes mellitus in Italy reported an acceptance rate of 85.80%. One study based in China reported a 77.4% vaccine-acceptance rate among pregnant women.
