3. Bioactive Compounds from Fungal Endophytes of Mangroves
Marine fungi are often involved in mutualistic interactions, as their survival may well require relationships including symbiosis with other organisms. Many endophytic fungi have been isolated from mangroves, which are intertidal wetland environments comprising closely associated plants, animals, and microbes from many genera. These environments line (sub)tropical habitats, comprising one-quarter of the tropical coastline in the world (over 15.5 million hectares)
[29][31].
Mangroves are frontiers between land and sea, a mixture of freshwater and saltwater, exporting plant detritus and faunal biomass to support life offshore. Currently, the Bandaranayake paper
[29][31] has been cited over 350 times, demonstrating the “ecological value” of these marine/freshwater environments. These environments are complex and rich in fungal species diversity, largely based on the availability of colonizable substrata. Within these environments, fungi play an important role in the nutrient cycles, transforming polymeric substances into smaller and simpler organic matter that can be used by other organisms
[30][32]. These secondary metabolic products help organisms cope with biotic stress factors, such as varying water and salt levels. There is also intense competition for space; thus, secondary metabolites are used as a form of chemical defense and/or offense to combat pathogens, herbivores, and other organisms.
Two recent reviews on anti-infectives from mangrove endophytic fungi were published by Deshmukh et al. in 2020
[31][33] and Cadamuro et al. in 2021
[32][34]. Many of these fungi have been reported from China, primarily due to their research groups now studying mangrove endophytes instead of their medicinal plant hosts
[33][35]. These fungi typically belong to the genera
Aspergillus,
Phomopsis,
Pestalotiopsis, and
Penicillium, which is not surprising due to the cosmopolitan nature of these genera. The Chinese have a longstanding history of using traditional herbal mixtures to treat disease and have known about the toxic properties of marine natural products for centuries. However, many of these newly isolated compounds have been published in local journals or those dedicated to plant research, such as Phytochemistry, Planta Medica, Phytomedicine, or Phytochemical letters, and thus were not frequently read/cited by scientists involved in marine chemistry.
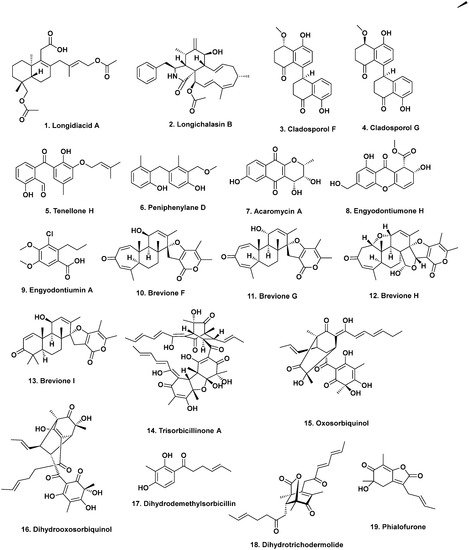
The She group at Sun Yat-Sen University has focused on identifying bioactive metabolites from mangrove-derived endophytic fungi from the South China Sea. Many of these metabolites have been published in Phytochemical Letters, Planta Medica, as well as Marine Drugs. For example, in 2014, Liu and coworkers published a paper in Planta Medica on three new vermistatin derivatives produced by a mangrove endophytic
Penicillium sp. HN29-3B1 isolated from the sea mango
Cerbera manghas in the South China Sea. With the exception of 5′-hydroxypenisimplicissin, both 6-demethylpenisimplicissin (
50) and 2′-epihydroxydihydro-vermistatin (
51) exhibited α-glucosidase inhibitory activity with IC
50 values of 9.5 and 8.0 μM, respectively
[34][36]. A year later, this group reported the production of pinazaphilones A and B, 4′-(
S)-(3,5-dihydroxyphenyl)-4′-hydroxy-6′-methylcyclopent-1′-en-5′-one, 6′-methyl-[1,1′-biphenyl]-3,3′,4′,5-tetraol, and penicidone D from the same fungal isolate. Pinazaphilone B (
52) and 6′-methyl-[1,1′-biphenyl]-3,3′,4′,5-tetraol (
53) inhibited α-glucosidase with IC
50 values of 28.0 and 2.2 μM, respectively, demonstrating their potential as antidiabetic agents
[35][37].
The She group has reported several metabolites produced by endophytic
Talaromyces. In 2011, they reported cytotoxic norsesquiterpene peroxides produced by an endophytic
T. flavus isolated from the leaves of the mangrove plant
Sonneratia apetala collected along the saltmarsh of the South China Sea
[36][38]. Talaperoxides A to D (
54–
57), constituted two isomeric pairs exhibiting toxicity to brine shrimp at median lethal doses (LD
50) of less than 10 ppm. These compounds were also evaluated for cytotoxicity against human breast (MCF-7 and MDA-MB-435), hepatoma (HepG2), cervical (HeLa), and prostate (PC-3) cancer cell lines. Talaperoxides B (
55) and D (
57) were cytotoxic against these cell lines with IC
50 values ranging from 0.89 to 1.92 μg/mL. The group later reported two new benzophenone derivatives, peniphenone and methylpeniphenone, from the mycelia of an endophytic
Penicillium sp. ZJ-SY2 isolated from the same plant
[37][39]. Peniphenone (
58) exhibited immunosuppressive activity against T-cell (concanavalin A-induced) and B-cell (liposaccharide-induced) proliferation in mouse splenic lymphocytes with IC
50 values of 8.1 and 9.3 μg/mL, respectively. The methyl ester methylpeniphenone (
59) exhibited weaker activity against T cells and B cells with IC
50 values of 17.5 and 23.7 μg/mL, respectively. More recently, the group reported two new depsidones, talaromyones A and B, produced by cultures of
Talaromyces stipitatus SK-4 isolated from the leaves of the mangrove plant
Acanthus ilicifolius from the Shankou Mangrove Nature Reserve in China. The acetylated depsidone talaromyone B (
60) exhibited antibacterial activity against
B. subtilis (MIC = 12.5 μg/mL) as well as α-glucosidase activity (IC
50 = 48.4 μM)
[38][40].
Over the last decade, reports on the number of mangrove-derived strains of
Talaromyces that produce bioactive metabolites have increased, especially as taxonomic revisions have been made to species with symmetrical biverticillate conidiophores. For more information on secondary metabolites from mangrove-associated strains of
Talaromyces, the 2018 review by Nicoletti et al. in Marine Drugs is an excellent source of information
[39][41].
Other research groups have reported bioactive compounds from fungal endophytes isolated from mangrove-associated medicinal plants. For example, Lin and coworkers published a paper in Phytochemistry describing four new polyketides produced by endophytic
Penicillium sp. JP-1 isolated from the inner bark of
Aegiceras corniculatum collected in Fujian, China
[40][42].
A. corniculatum is a shrub known for its analgesic, cytotoxic, and antidiabetic properties
[41][42][43,44]. Leptosphaerone C, penicillenone, arugosin I, and 9-demethyl FR-901235 isolated from fungal endophytes of this plant were evaluated for cytotoxic activity against human lung cancer (A-549) and murine lymphocytic leukemia (P388) cell lines. Leptosphaerone C (
61) exhibited cytotoxic activity against human lung cancer cells (A-549; IC
50 1.45 μM) while penicillenone (
62) was cytotoxic against murine lymphocytic leukemia (P388; IC
50 = 1.38 μM).
In 2012, the Wang group published a communication reporting a new cytotoxic and antifungal metabolite, chaetoglobosin X (
63) from an endophytic fungus isolated from the leaves of
Curcuma wenyujin Y.H. Chen et C. Ling, which belongs to the ginger family, collected in Zhejiang Province, Wenzhou, China
[43][45]. Chaetoglobosin X (
63) exhibited antifungal activity against several plant pathogens, including
Exerohilum turcicum,
F. oxysporum f. sp.
Cucumerinim,
Curvularia lunata (MIC; 3.125 μg/mL), as well as
F. graminearum and
F. moniliforme (MIC; 6.25 μg/mL). Other chaetoglobosins have been reported from the mangrove endophytic fungus
P. chrysogenum V11 isolated from the vein of the medicinal plant
Myoporum bonitioides A. Gray in the Leizhou Penninsula in China
[44][46]. These included penochalasin I (
64), containing an unprecedented six-membered fused ring system, and penochalasin J (
65). Penochalasin I (
65) exhibited cytotoxic activity against gastric (SGC-7901) and breast cancer cell lines (MDA-MB-435) with IC
50 values below 10 μM. Penochalasin J (
65) exhibited antifungal activity against the plant pathogen
Colletotrichum gloeosporioides with an MIC of 25.08 μM, which was more active than the known antifungal agent carbendazim. Other antifungal agents have been reported from endophytic fungi from this plant. These agents can be found in the following reports by Li et al.
[45][46][47][47,48,49].
4. Bioactive Compounds from Fungi Isolated from Marine Vertebrates and Selected Invertebrates
In addition to mangrove plants, marine fungi have mutualistic interactions with other organisms, including marine vertebrates, animals with backbones including fish, amphibians, mammals, reptiles, and birds. These animals have commensal, competitive, and predatory interactions with each other. For example, mammals find food by following birds and birds take advantage of the herding efforts of predatory fishes and mammals for food. Furthermore, these animals are active and mobile, covering wide distances to avoid competitors, reducing the rate of evolutionary divergence. Yet, their mobility also provides opportunities to obtain nutrients from different locations, increasing the diversity of their microbiomes.
From initial sequencing studies, the microbiomes of some mammals and marine vertebrates were found, as expected, to have gut microbiota diversity across species
[48][49][50][51][59,60,61,62]. Several fish microbiomes have been sequenced and found to be unique environmental niches for the microbial production of new bioactive molecules
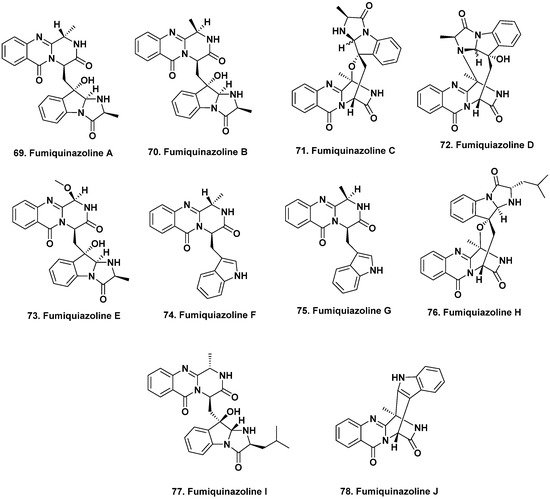
[52][63]. While fish are the most dominant marine vertebrates, only a small number of natural products have so far been reported from these organisms. Fumiquinazolines A–G (
69–
75) were produced by the fungus
A. fumigatus isolated from the gastrointestinal tract of the Japanese saltwater fish
Pseudolabrus japonicas [53][54][64,65]. These peptidyl alkaloids have variable degrees of oxygenation, methylation, and substitution on their indole moieties and all exhibited cytotoxic activity against the murine lymphocytic leukemia (P388) cells with ED
50 values of 6.1, 16.0, 14.6, 17.7, 52.0, 13.5 and 13.8 μg/mL, respectively.
This next section might seem out of place, but we have put it here due to the similarity in the structures that were isolated, as this demonstrates that the “nominal host” is perhaps not the controlling factor. The structurally related fumiquinazolines H–L were isolated from fermentation of an
Acremonium sp. obtained from the
non-vertebrate marine tunicate
Ecteinascidia turbinata [55][66], as well as other marine-derived
A. fumigatus and endophytic
Scopulariopsis sp. isolated from
non-vertebrate gorgonians
[56][67]. Some of these fumiquinazolines inhibited the proliferation of mouse CDC2-mutant (tsFT210) cells, including compounds
69,
71, and
74 from the fish-related fungus and fumiquinazoline J (
78,
Figure 6) from the tunicate, while fumiquinazolines H–I (
76,
77) exhibited antifungal activity. The 2019 review by Resende et al.
[57][68] is an excellent discussion on the fumiquinazolines, covering their source(s), chemical and biological activities. This review along with the 2020 article in Marine Drugs by Han et al.
[58][69] should be consulted for current information on this class of fungal metabolites and relatives, including genomic aspects underlying their production.
Figure 6. Bioactive Fumiquinazolines from Vertebrates and Invertebrates (Structures
69 to
78).
Due to the duplication of structures and names related to fumiquinazolines published from different sources, with approximately 80 mentioned in the Resende et al. review
[57][68], we have used the structures for fumiquinazoline A to J from that source, rather than those first reported in 1992
[53][64].