Dry reforming of hydrocarbons (DRH) is a pro-environmental method for syngas production. It owes its pro-environmental character to the use of carbon dioxide, which is one of the main greenhouse gases. Transition metal carbides (TMCs) can potentially replace traditional nickel catalysts due to their stability and activity in DR processes.
- dry reforming
- catalysts
- metal carbides
- molybdenum
- tungsten
- titanium
1. Introduction
| Process | Main Reaction | Enthalpy ΔH0298 K [kJ/mol] | Pressure [bar] | H2/CO Ratio |
|---|---|---|---|---|
| Dry reforming of methane (DRM) | CH4 + CO2 = 2CO + 3 H2 | +247 | 1 | 1:1 |
| Steam reforming of methane (STM) | CH4 + H2O = CO + 3H2O | +206 | 3–25 | 3:1 |
| Partial oxidation of methane (POM) | CH4 + ½ O2 = CO + 2H2 | −35.2 | 100 | 2:1 |
| Autothermal reforming (ATR) | CH4 + H2O = CO + 3H2O CH4 + ½ O2 = CO + 2H2 |
+206 −35.2 |
1–50 | 1:1 or 1:2 |
2. Metal Carbides
2.1. Tungsten Carbide
2.2. Molybdenum Carbide
2.3. Titanium Carbide
3. The Use of Metal Carbides for Dry Reforming
3.1. Tungsten Carbide
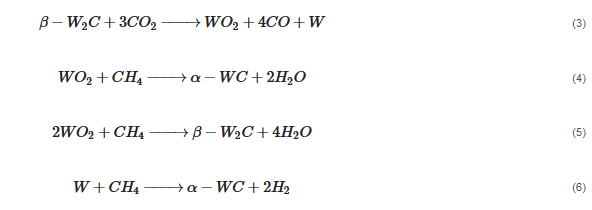

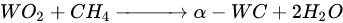

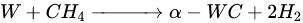



5.2. WC Combined with Nickel and Cobalt Particles
3.2. WC Combined with Nickel and Cobalt Particles
Tungsten carbide is used as a catalyst in dry methane reforming, usually in combination with nickel or cobalt, because the addition of a second metal can modify the catalytic performance and structure of this carbide [204,205][45][46]. Despite the unique properties of WC, this compound has a surface with a strong oxygen affinity. As a consequence, this leads to blockage of the surface in the event of irreversible adsorption of oxygen-containing substances, which, in turn, results in a reduction in catalytic activity [206][47]. Therefore, to avoid this problem, core–shell systems are used, that is, WC cores covered with a metallic coating that prevents oxidation of the carbide surface, thus promoting structural stability [207][48]. Co-WC and Ni-WC are stable, active, and selective catalysts in dry methane reforming [208][49].
According to Barbosa et al., higher CO2 conversion values compared to CH4 conversion in dry methane reforming are obtained using Ni-Mo2C and Ni-WC catalysts [209][50]. This is probably due to the reactions occurring, including the Boudouard reaction (Equation (97)), as a result of which the forming CO2 is activated in the carbide (Equation (108)), leading to the oxidation of WC (Equations (119) and (120)) and the conversion of CO with steam (Equation (131)), in which part of the hydrogen obtained reacts with CO2, resulting in a lower H2/CO ratio and in increased CO2 conversion. However, regardless of the presence of nickel and the Ni/W ratio, the less stable β-W2C is transformed into α-WC during dry methane reforming, according to Equations (3)–(6) [198][39].
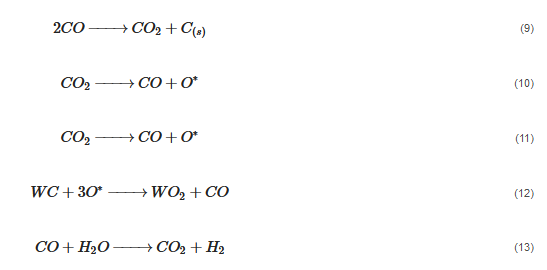
(7)
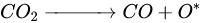
(8)
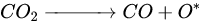
(9)

(10)

(11)
In the case of cobalt tungsten carbide (Co6W6C), the addition of carbon in the early stages of the catalytic reaction results in the conversion of the bimetallic carbide to a stable form containing active sites for dry methane reforming [205][46], according to Equation (142).
5.3. Molybdenum Carbide

(12)
3.3. Molybdenum Carbide
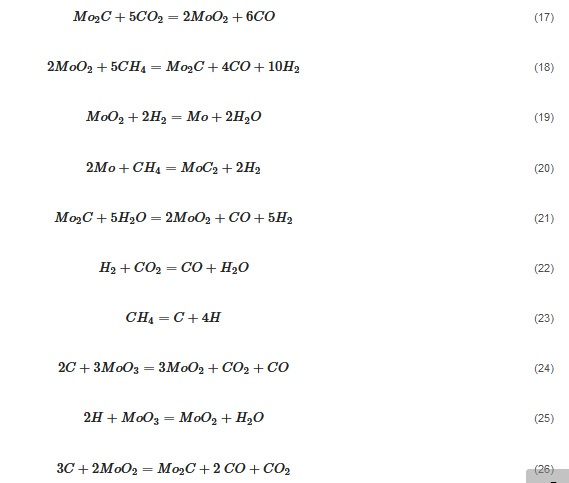
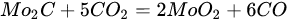
(13)
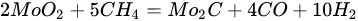
(14)
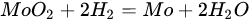
(15)
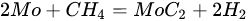
(16)
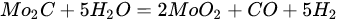
(17)
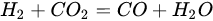
(18)
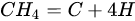
(19)
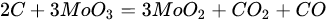
(20)
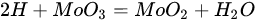
(21)
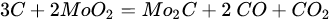
(22)
An overwhelming number of research reports on the catalytic activity of molybdenum carbides in the dry reforming of hydrocarbons refer to catalysts prepared using TPR method. The physicochemical properties and resulting catalytic activity of molybdenum carbide catalysts are influenced by the molybdenum-to-carbon ratio. Gao et al. [79][55] reported a series of molybdenum carbide catalysts that differ in the weight content of Mo in order to use carbon nanotubes as a carbon source (Mo 0, 5, 10, 15, 30, 60, and 100 wt.%). Along with an increasing proportion of molybdenum in the catalyst, a decrease in the specific surface area, diameter, and pore volume was observed. A correlation was observed between the molybdenum content and catalytic activity in the dry methane reforming process. The highest activity was observed for the catalyst containing 30 wt.% of Mo. Another of the key structural parameters of the carbide for catalytic activity is the excess unbound carbon formed during the synthesis process. Roohi et al. [216][56] found that the amount of excess carbon depends on the carburization temperature and the concentration of carbon-containing gas during the synthesis. Catalysis with lower contents of excess carbon exhibited an initial higher activity in the DRM reaction; however, during the long-term test, the molybdenum loading was a crucial factor. Several articles have been published to investigate the effect of the crystal structure on catalytic activity in DRM [78,217][57][58]. Liang et al. [78][57] investigated the catalytic activity of β-Mo2C and α-MoC1-x phases in DRM. Both phases were characterized by a narrow size distribution of up to 5 nm. Better activity was observed for the -MoC1-x phase. Oshikawa et al. [217][58] observed the dependence of the η-Mo3C2 phase on the methane decomposition rate. They reported the key role of the η-Mo3C2 phase among other molybdenum carbide phases as an active species for methane reforming. During the DRM process, the molybdenum carbide may be partially oxidized to the form of an oxycarbide. Kurlov et al. [218][59] reported that the oxycarbidic phase Mo2CxOy exhibits high stability toward further oxidation to MoO2, and the increase in β-Mo2C/ Mo2CxOy active sites correlates with higher efficiency in the DRM reaction.5.4. Molybdenum Carbide Modified with Nickel Particles
3.4. Molybdenum Carbide Modified with Nickel Particles
Molybdenum carbide catalysts during DRM at atmospheric pressure may suffer from deactivation due to oxidation with carbon dioxide. The carbide structure is reconstructed with the carbon element from the dissociation of methane; however, oxidation with CO2 is more favorable [219][60]. The combination of molybdenum carbide with other metals: Ni [222][61], Co [223][62], and Fe [24][63], allows controlled dissociation paths of CO2 and CH4, ensuring appropriate conditions for oxidation–recarburization cycles [47,224][16][64]. The introduction of other metals into the carbide catalyst results in the generation of more moles of hydrogen, leading to a higher H2/CO ratio in the outlet stream. Carbide and the introduced metal (Ni, Co) act as an active center for the dissociation of CO2 and methane, respectively. It is generally accepted that the catalytic activity of nickel catalysts is strictly connected with the size of the nickel particles: the smaller the Ni particles, the better the catalytic activity, resulting from the stronger active metal–support interactions, delayed sintering, and a lower rate of formation of carbon deposits [3,5,14,225][3][5][65][66]. However, in the case of molybdenum carbide supported nickel catalysts, the ratio of Ni/Mo to the size of nickel particles plays a predominant role [51,226][19][67]. The nickel-to-molybdenum ratio affects the morphology and catalytic activity of Mo2C. Moreover, too high a dissociation of CH4 promotes the formation of coke on the surface of the catalysts [79,222][55][61]. Zhang et al. [222][61] observed that with an increasing nickel content in nickel-modified Mo2C supported on carbon nanotubes, the crystallite size of Mo2C for Ni/Mo ratios = 0.5, 1, 1.5, and 2 was equal to 53, 38, 35, and 28 nm, respectively. Moreover, the increase in the Ni content resulted in an increase in the particle size. Catalytic activity increased with an increasing Ni/Mo ratio to the optimal value (1:1). After this value was exceeded, the activity decreased despite the higher content and smaller particle size of nickel. The DRM process is carried out mainly at temperatures above 800 °C. The performance of processes at lower temperatures results in lower methane and carbon dioxide conversions, as well as a lower H2/CO ratio [119,123][68][69]. However, Diao et al. [227][70] recently reported the high catalytic activity of a Ni-Mo2C/Al2O3 catalyst at 470 °C in a catalytic bed coupled with non-thermal plasma treatment. The molybdenum-nickel-alumina catalyst exhibited superior activity compared to Ni/Al2O3. The H2/CO ratio was equal to 0.9, and the conversions of CH4 and CO2 were around 80% and 85%, respectively. Both bare and nickel-modified molybdenum carbide catalysts are used, both supported and unsupported. Deposition on an inert substrate allows for dilution of the catalyst, thus eliminating channeling, and retarding heat transfer limitations and pressure drop across the catalytic bed [194][34]. As a support, metal oxides: La2O3 [224][64], Al2O3 [12[71][72][67],121,226], SiO2 [10], ZrO2 [116][73], MgO [228][74], biochar [77][75], carbon nanotubes [222][61], zeolites [26][76], and silicon carbide [10], have been examined. Silva et al. [10] investigated the effect of the support (SiO2, Al2O3, and SiC) Ni-Mo2C on catalytic activity and stability in the DRM reaction. The lowest DRM substrate conversions and H2/CO ratios were observed for the silica support. As a reason for the low activity observed for the SiO2-supported samples, there were weak interactions between Ni and SiO2, leading to movement of Ni species at the surface of the catalysts, retarding the interface contact between Ni and Mo2C responsible for the oxidation–recarburization cycle, Ni aggregates, and the formation of filamentous carbon.5.5. MAX and MXenes for Dry Reforming of Hydrocarbons
3.5. MAX and MXenes for Dry Reforming of Hydrocarbons
A special family of transition metal carbides is constituted by multilayer metal carbides with a 2D nanosheet structure similar to that of graphene, belonging to the group of compounds called MXenes. The term MXenes denotes carbides and nitrides of transition metals, with the general formula Mn+1XnTx, where n = 1, 2, 3, or 4, M refers to the transition metal (M = Sc, Ti, V, Cr, Mn, Y, Zr, Nb, Mo, Hf, Ta, and W [23,161][77][78]), and X refers to the p-block element (silicon, aluminum, gallium), while T describes the type of terminal groups (–O, –OH, –F, –Cl) in the amount of x per selected unit. They are obtained by selectively removing component A from the ternary MAX matrix. The MAX matrix consists of the elements of the transition metal M, a p group element (A), and carbon or nitrogen (X). MXene compounds are gaining importance due to their metal-like conductive properties, thermal and chemical stability, and the ability to manipulate properties through simple and effective modification of terminal groups [162,163][79][80]. Their unique properties allow for application in various branches of science: energy storage [163[80][81],164], electrocatalysis [165[82][83],166], photocatalysis [167[84][85],168], and heterogeneous catalysis [23,164,165,166,169][77][81][82][83][86].
References
- Pakhare, D.; Spivey, J. A Review of Dry (CO2) Reforming of Methane over Noble Metal Catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837.
- Yentekakis, I.V.; Goula, G. Biogas Management: Advanced Utilization for Production of Renewable Energy and Added-Value Chemicals. Front. Environ. Sci. 2017, 5, 7.
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry Reforming of Methane: Influence of Process Parameters—A Review. Renew. Sustain. Energy Rev. 2015, 45, 710–744.
- Guharoy, U.; Reina, T.R.; Liu, J.; Sun, Q.; Gu, S.; Cai, Q. A Theoretical Overview on the Prevention of Coking in Dry Reforming of Methane Using Non-Precious Transition Metal Catalysts. J. CO2 Util. 2021, 53, 101728.
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A Review of Recent Efforts to Promote Dry Reforming of Methane (DRM) to Syngas Production via Bimetallic Catalyst Formulations. Appl. Catal. B Environ. 2021, 296, 120210.
- Mohamedali, M.; Henni, A.; Ibrahim, H. Recent Advances in Supported Metal Catalysts for Syngas Production from Methane. ChemEngineering 2018, 2, 9.
- Arora, S.; Prasad, R. An Overview on Dry Reforming of Methane: Strategies to Reduce Carbonaceous Deactivation of Catalysts. RSC Adv. 2016, 6, 108668–108688.
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A Review on Catalyst Development for Dry Reforming of Methane to Syngas: Recent Advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193.
- Ma, Y.; Guan, G.; Hao, X.; Cao, J.; Abudula, A. Molybdenum Carbide as Alternative Catalyst for Hydrogen Production—A Review. Renew. Sustain. Energy Rev. 2017, 75, 1101–1129.
- Silva, C.G.; Passos, F.B.; da Silva, V.T. Influence of the Support on the Activity of a Supported Nickel-Promoted Molybdenum Carbide Catalyst for Dry Reforming of Methane. J. Catal. 2019, 375, 507–518.
- Jewkes, J.; Sawers, D.; Stillerman, R. The Sources of Invention. In Tungsten Carbides; Palgrave Macmillan: London, UK, 1969; pp. 319–320.
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tungsten-carbide#section=Boiling-Point (accessed on 5 July 2021).
- Yan, Z.; Cai, M.; Shen, P.K. Nanosized Tungsten Carbide Synthesized by a Novel Route at Low Temperature for High Performance Electrocatalysis. Sci. Rep. 2013, 3, 1646.
- Levy, R.B.; Boudart, M. Platinum-like Behavior of Tungsten Carbide in Surface Catalysis. Science 1973, 181, 547–549.
- Rasaki, S.A.; Zhang, B.; Anbalgam, K.; Thomas, T.; Yang, M. Synthesis and Application of Nano-Structured Metal Nitrides and Carbides: A Review. Prog. Solid State Chem. 2018, 50, 1–15.
- Shi, C.; Zhang, A.; Li, X.; Zhang, S.; Zhu, A.; Ma, Y.; Au, C. Ni-Modified Mo2C Catalysts for Methane Dry Reforming. Appl. Catal. A Gen. 2012, 431–432, 164–170.
- Alaba, P.A.; Abbas, A.; Huang, J.; Daud, W.M.A.W. Molybdenum Carbide Nanoparticle: Understanding the Surface Properties and Reaction Mechanism for Energy Production towards a Sustainable Future. Renew. Sustain. Energy Rev. 2018, 91, 287–300.
- Vasilevich, A.V.; Baklanova, O.N.; Lavrenov, A.V. Molybdenum Carbides: Synthesis and Application in Catalysis. Solid Fuel Chem. 2020, 54, 354–361.
- Lohse, B.H.; Calka, A.; Wexler, D. Effect of Starting Composition on the Synthesis of Nanocrystalline TiC during Milling of Titanium and Carbon. J. Alloys Compd. 2005, 394, 148–151.
- Vidal, A.B.; Feria, L.; Evans, J.; Takahashi, Y.; Liu, P.; Nakamura, K.; Illas, F.; Rodriguez, J.A. CO2 Activation and Methanol Synthesis on Novel Au/TiC and Cu/TiC Catalysts. J. Phys. Chem. Lett. 2012, 3, 2275–2280.
- Rodriguez, J.A.; Evans, J.; Feria, L.; Vidal, A.B.; Liu, P.; Nakamura, K.; Illas, F. CO2 Hydrogenation on Au/TiC, Cu/TiC, and Ni/TiC Catalysts: Production of CO, Methanol, and Methane. J. Catal. 2013, 307, 162–169.
- Back, S.; Jung, Y. TiC- and TiN-Supported Single-Atom Catalysts for Dramatic Improvements in CO2 Electrochemical Reduction to CH4. ACS Energy Lett. 2017, 2, 969–975.
- Huang, T.; Fang, H.; Mao, S.; Yu, J.; Qi, L. In-Situ Synthesized as High-Performance Catalysts for Oxygen Reduction Reaction. Carbon 2018, 126, 566–573.
- Yue, R.; Xia, M.; Wang, M.; Chen, P.; Gong, W.M.; Liao, S.; Li, Z.; Gao, F.; Zhang, L.; Wang, J. TiN and TiC as Stable and Promising Supports for Oxygen Reduction Reaction: Theoretical and Experimental Study. Appl. Surf. Sci. 2019, 495, 143620.
- Rodriguez, J.A.; Ramírez, P.J.; Gutierrez, R.A. Highly Active Pt/MoC and Pt/TiC Catalysts for the Low-Temperature Water-Gas Shift Reaction: Effects of the Carbide Metal/Carbon Ratio on the Catalyst Performance. Catal. Today 2017, 289, 47–52.
- Wang, Y.; Zhang, X.; Cheng, C.; Yang, Z. TiC Supported Single-Atom Platinum Catalyst for CO Oxidation: A Density Functional Theory Study. Appl. Surf. Sci. 2018, 453, 159–165.
- Sahoo, S.K.; Ye, Y.; Lee, S.; Park, J.; Lee, H.; Lee, J.; Han, J.W. Rational Design of TiC-Supported Single-Atom Electrocatalysts for Hydrogen Evolution and Selective Oxygen Reduction Reactions. ACS Energy Lett. 2019, 4, 126–132.
- Xu, L.; Miao, Z.; Song, H.; Chen, W.; Chou, L. Significant Roles of Mesostructure and Basic Modifier for Ordered Mesoporous Ni/CaO-Al2O3 Catalyst towards CO2 Reforming of CH4. Catal. Sci. Technol. 2014, 4, 1759–1770.
- Wang, N.; Shen, K.; Yu, X.; Qian, W.; Chu, W. Preparation and Characterization of a Plasma Treated NiMgSBA-15 Catalyst for Methane Reforming with CO2 to Produce Syngas. Catal. Sci. Technol. 2013, 3, 2278–2287.
- Mo, L.; Leong, K.K.M.; Kawi, S. A Highly Dispersed and Anti-Coking Ni-La2O3/SiO2 Catalyst for Syngas Production from Dry Carbon Dioxide Reforming of Methane. Catal. Sci. Technol. 2014, 4, 2107–2114.
- Brungs, A.J.; York, A.P.E.; Green, M.L.H. Comparison of the Group V and VI Transition Metal Carbides for Methane Dry Reforming and Thermodynamic Prediction of Their Relative Stabilities. Catal. Lett. 1999, 57, 65–69.
- Wang, X.H.; Zhang, M.H.; Li, W.; Tao, K.Y. Synthesis and Characterization of Cobalt-Molybdenum Bimetallic Carbides Catalysts. Catal. Today 2008, 131, 111–117.
- Yao, Z.; Jiang, J.; Zhao, Y.; Luan, F.; Zhu, J.; Shi, Y.; Gao, H.; Wang, H. Insights into the Deactivation Mechanism of Metal Carbide Catalysts for Dry Reforming of Methane via Comparison of Nickel-Modified Molybdenum and Tungsten Carbides. RSC Adv. 2016, 6, 19944–19951.
- Lamont, D.C.; Thomson, W.J. Dry Reforming Kinetics over a Bulk Molybdenum Carbide Catalyst. Chem. Eng. Sci. 2005, 60, 3553–3559.
- Erdöhelyi, A.; Cserényi, J.; Papp, E.; Solymosi, F. Catalytic Reaction of Methane with Carbon Dioxide over Supported Palladium. Appl. Catal. A Gen. 1994, 108, 205–219.
- Mounfield, W.P.; Harale, A.; Román-Leshkov, Y. Impact of Morphological Effects on the Activity and Stability of Tungsten Carbide Catalysts for Dry Methane Reforming. Energy Fuels 2019, 33, 5544–5550.
- Zhang, Q.; Pastor-Pérez, L.; Gu, S.; Reina, T.R. Transition Metal Carbides (TMCS) Catalysts for Gas Phase CO2 Upgrading Reactions: A Comprehensive Overview. Catalysts 2020, 10, 955.
- Shao, H.; Kugler, E.L.; Dadyburjor, D.B. Ni-W-C and Co-W-C as Alternative Catalysts for Dry Reforming and Steam Reforming of Methane. Carbon 2006, 2712, 26506.
- Zhang, Y.; Zhang, S.; Zhang, X.; Qiu, J.; Yu, L.; Shi, C. Ni Modified WC x Catalysts for Methane Dry Reforming. In Advances in CO2 Capture, Sequestration, and Conversion; American Chemical Society: Washington, DC, USA, 2015; pp. 171–189.
- Yan, Q.; Lu, Y.; To, F.; Li, Y.; Yu, F. Synthesis of Tungsten Carbide Nanoparticles in Biochar Matrix as a Catalyst for Dry Reforming of Methane to Syngas. Catal. Sci. Technol. 2015, 5, 3270–3280.
- Hunt, S.T.; Kokumai, T.M.; Zanchet, D.; Román-Leshkov, Y. Alloying Tungsten Carbide Nanoparticles with Tantalum: Impact on Electrochemical Oxidation Resistance and Hydrogen Evolution Activity. J. Phys. Chem. C 2015, 119, 13691–13699.
- Vijayakumar, P.; Senthil Pandian, M.; Lim, S.P.; Pandikumar, A.; Huang, N.M.; Mukhopadhyay, S.; Ramasamy, P. Facile Synthesis of Tungsten Carbide Nanorods and Its Application as Counter Electrode in Dye Sensitized Solar Cells. Mater. Sci. Semicond. Process. 2015, 39, 292–299.
- Michalsky, R.; Zhang, Y.J.; Medford, A.J.; Peterson, A.A. Departures from the Adsorption Energy Scaling Relations for Metal Carbide Catalysts. J. Phys. Chem. C 2014, 118, 13026–13034.
- Michalsky, R.; Zhang, Y.J.; Peterson, A.A. Trends in the Hydrogen Evolution Activity of Metal Carbide Catalysts. ACS Catal. 2014, 4, 1274–1278.
- Xiao, T.; Wang, H.; York, A.P.E.; Williams, V.C.; Green, M.L.H. Preparation of Nickel-Tungsten Bimetallic Carbide Catalysts. J. Catal. 2002, 209, 318–330.
- Shao, H.; Kugler, E.L.; Ma, W.; Dadyburjor, D.B. Effect of Temperature on Structure and Performance of In-House Cobalt-Tungsten Carbide Catalyst for Dry Reforming of Methane. Ind. Eng. Chem. Res. 2005, 44, 4914–4921.
- Hunt, S.T.; Milina, M.; Alba-Rubio, A.C.; Hendon, C.H.; Dumesic, J.A.; Roman-Leshkov, Y. Self-Assembly of Noble Metalmonolayers on Transition Metalcarbide Nanoparticle Catalysts. Science 2016, 352, 974–978.
- Wannakao, S.; Artrith, N.; Limtrakul, J.; Kolpak, A.M. Engineering Transition-Metal-Coated Tungsten Carbides for Efficient and Selective Electrochemical Reduction of CO2 to Methane. ChemSusChem 2015, 8, 2745–2751.
- Li, Z.S.; Cai, N.S. Modeling of Multiple Cycles for Sorption-Enhanced Steam Methane Reforming and Sorbent Regeneration in Fixed Bed Reactor. Energy Fuels 2007, 21, 2909–2918.
- Barbosa, R.D.; Baldanza, M.A.S.; de Resende, N.S.; Passos, F.B.; da Silva, V.L.d.S.T. Nickel–Promoted Molybdenum or Tungsten Carbides as Catalysts in Dry Reforming of Methane: Effects of Variation in CH4/CO2 Molar Ratio. Catal. Lett. 2021, 151, 1578–1591.
- Rase, H.F.; Maddox, L.A. Titanium Carbide Catalysts, and the Catalyst Compositions. U.S. Patent 3,865,750, 11 February 1971.
- Xie, Z.; Deng, Y.; Yang, Y.; Su, H.; Zhou, D.; Liu, C.; Yang, W. Preparation of Nano-Sized Titanium Carbide Particles via a Vacuum Carbothermal Reduction Approach Coupled with Purification under Hydrogen/Argon Mixed Gas. RSC Adv. 2017, 7, 9037–9044.
- Gou, H.P.; Zhang, G.H.; Chou, K.C. Formation of Submicrometer Titanium Carbide from a Titanium Dioxide Encapsulated in Phenolic Resin. J. Mater. Sci. 2016, 51, 7008–7015.
- Solymosi, F.; Németh, R.; Oszkó, A. The Oxidative Dehydrogenation of Propane with CO2 over Supported Mo2C Catalyst. Stud. Surf. Sci. Catal. 2001, 136, 339–344.
- Gao, H.; Yao, Z.; Shi, Y.; Jia, R.; Liang, F.; Sun, Y.; Mao, W.; Wang, H. Simple and Large-Scale Synthesis of β-Phase Molybdenum Carbides as Highly Stable Catalysts for Dry Reforming of Methane. Inorg. Chem. Front. 2018, 5, 90–99.
- Roohi, P.; Alizadeh, R.; Fatehifar, E. Dry Reforming of Methane over Nano-Mo2C/Al2O3 Catalyst: Effect of Carburization Conditions on Excess Carbon Deposition. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 38, 3565–3571.
- Liang, P.; Gao, H.; Yao, Z.; Jia, R.; Shi, Y.; Sun, Y.; Fan, Q.; Wang, H. Simple Synthesis of Ultrasmall β-Mo2C and α-MoC1-x Nanoparticles and New Insights into Their Catalytic Mechanisms for Dry Reforming of Methane. Catal. Sci. Technol. 2017, 7, 3312–3324.
- Oshikawa, K.; Nagai, M.; Omi, S. Active Species of Molybdenum Carbide Catalysts in Methane Reforming: η-Mo3C2. Chem. Lett. 2000, 29, 1086–1087.
- Kurlov, A.; Huang, X.; Deeva, E.B.; Abdala, P.M.; Fedorov, A.; Müller, C.R. Molybdenum Carbide and Oxycarbide from Carbon-Supported MoO3 Nanosheets: Phase Evolution and DRM Catalytic Activity Assessed by TEM and: In Situ XANES/XRD Methods. Nanoscale 2020, 12, 13086–13094.
- Guo, J.; Zhang, A.J.; Zhu, A.M.; Xu, Y.; Au, C.T.; Shi, C. A Carbide Catalyst Effective for the Dry Reforming of Methane at Atmospheric Pressure. ACS Symp. Ser. 2010, 1056, 181–196.
- Zhang, L.; Yang, Y.; Yao, Z.; Yan, S.; Kang, X. Finding of a New Cycle Route in Ni/Mo2C Catalyzed CH4-CO2 reforming. Catal. Sci. Technol. 2021, 11, 479–483.
- Du, X.; France, L.J.; Kuznetsov, V.L.; Xiao, T.; Edwards, P.P.; AlMegren, H.; Bagabas, A. Dry Reforming of Methane over ZrO2-Supported Co–Mo Carbide Catalyst. Appl. Petrochem. Res. 2014, 4, 137–144.
- Lalsare, A.D.; Leonard, B.; Robinson, B.; Sivri, A.C.; Vukmanovich, R.; Dumitrescu, C.; Rogers, W.; Hu, J. Self-Regenerable Carbon Nanofiber Supported Fe–Mo2C Catalyst for CH4-CO2 Assisted Reforming of Biomass to Hydrogen Rich Syngas. Appl. Catal. B Environ. 2021, 282, 119537.
- Zhang, S.; Shi, C.; Chen, B.; Zhang, Y.; Zhu, Y.; Qiu, J.; Au, C. Catalytic Role of β-Mo2C in DRM Catalysts That Contain Ni and Mo. Catal. Today 2015, 258, 676–683.
- Ghasali, E.; Ebadzadeh, T.; Alizadeh, M.; Razavi, M. Mechanical and Microstructural Properties of WC-Based Cermets: A Comparative Study on the Effect of Ni and Mo Binder Phases. Ceram. Int. 2018, 44, 2283–2291.
- Sugiyama, S.; Oribe, K.; Endo, S.; Yoshida, T.; Shimoda, N.; Katoh, M.; Kato, Y.; Ninomiya, W. Enhancement of the Catalytic Activity Associated with Carbon Deposition Formed on NiO/γ-Al2O3 Catalysts during the Direct Dehydrogenation of Isobutane. J. Chem. Eng. Jpn. 2021, 54, 35–43.
- Thakur, R.; Vahidmohammadi, A.; Smith, J.; Hoffman, M.; Moncada, J.; Beidaghi, M.; Carrero, C.A. Insights into the Genesis of a Selective and Coke-Resistant MXene-Based Catalyst for the Dry Reforming of Methane. ACS Catal. 2020, 10, 5124–5134.
- Duan, Y.; Shang, R.; Zhong, X.; Xie, W.; Wang, X.; Huang, L. In-Situ Synthesis of Ni-Mo2C/Al2O3 Catalysts for Dry Reforming of Methane. Int. J. Hydrogen Energy 2016, 41, 21955–21964.
- Gavrilova, N.; Dyakonov, V.; Myachina, M.; Nazarov, V.; Skudin, V. Synthesis of Mo2C by Thermal Decomposition of Molybdenum Blue Nanoparticles. Nanomaterials 2020, 10, 2053.
- Diao, Y.; Zhang, X.; Liu, Y.; Chen, B.; Wu, G.; Shi, C. Plasma-Assisted Dry Reforming of Methane over Mo2C-Ni/Al2O3 Catalysts: Effects of β-Mo2C Promoter. Appl. Catal. B Environ. 2022, 301, 120779.
- Wysocka, I.; Mielewczyk-Gryń, A.; Łapiński, M.; Cieślik, B.; Rogala, A. Effect of Small Quantities of Potassium Promoter and Steam on the Catalytic Properties of Nickel Catalysts in Dry/Combined Methane Reforming. Int. J. Hydrogen Energy 2021, 46, 3847–3864.
- Chi, J.Q.; Gao, W.K.; Lin, J.H.; Dong, B.; Qin, J.F.; Liu, Z.Z.; Liu, B.; Chai, Y.M.; Liu, C.G. Porous Core-Shell N-Doped Mo2 Nanospheres Derived from Inorganic-Organic Hybrid Precursors for Highly Efficient Hydrogen Evolution. J. Catal. 2018, 360, 9–19.
- Ren, P.; Zhao, Z. Unexpected Coke-Resistant Stability in Steam-CO2 Dual Reforming of Methane over the Robust Mo2C-Ni/ZrO2 Catalyst. Catal. Commun. 2019, 119, 71–75.
- Han, B.; Zhong, J.; Li, W.; Zhang, Z.; Bi, G.; Xie, J. The Promotional Role of β-Cyclodextrin on Ni-Mo2C/MgO Catalyst for Biogas Reforming. Mol. Catal. 2021, 515, 111897.
- Li, R.; Shahbazi, A.; Wang, L.; Zhang, B.; Chung, C.C.; Dayton, D.; Yan, Q. Nanostructured Molybdenum Carbide on Biochar for CO2 Reforming of CH4. Fuel 2018, 225, 403–410.
- Hodala, J.L.; Kotni, S.; Ramachandrarao, B.; Chelliahn, B. Metal Carbide as a Potential Non Noble Metal Catalyst for Naphtha Reforming. Fuel 2021, 288, 119610.
- Morales-Garciá, Á.; Calle-Vallejo, F.; Illas, F. MXenes: New Horizons in Catalysis. ACS Catal. 2020, 10, 13487–13503.
- Fu, L.; Xia, W. MAX Phases as Nanolaminate Materials: Chemical Composition, Microstructure, Synthesis, Properties, and Applications. Adv. Eng. Mater. 2021, 23, 2001191.
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Progress in Materials Science Characterization of MXenes at Every Step, from Their Precursors to Single Flakes and Assembled Films. Prog. Mater. Sci. 2021, 120, 100757.
- Chaudhari, N.K.; Jin, H.; Kim, B.; San Baek, D.; Joo, S.H.; Lee, K. MXene: An Emerging Two-Dimensional Material for Future Energy Conversion and Storage Applications. J. Mater. Chem. A 2017, 5, 24564–24579.
- Zhang, C.; Ma, Y.; Zhang, X.; Abdolhosseinzadeh, S.; Sheng, H.; Lan, W.; Pakdel, A.; Heier, J.; Nüesch, F. Two-Dimensional Transition Metal Carbides and Nitrides (MXenes): Synthesis, Properties, and Electrochemical Energy Storage Applications. Energy Environ. Mater. 2020, 3, 29–55.
- Meshkian, R.; Dahlqvist, M.; Lu, J.; Wickman, B.; Halim, J.; Thörnberg, J.; Tao, Q.; Li, S.; Intikhab, S.; Snyder, J.; et al. W-Based Atomic Laminates and Their 2D Derivative W1.33C MXene with Vacancy Ordering. Adv. Mater. 2018, 30, 1706409.
- Tao, Q.; Dahlqvist, M.; Lu, J.; Kota, S.; Meshkian, R.; Halim, J.; Palisaitis, J.; Hultman, L.; Barsoum, M.W.; Persson, P.O.Å.; et al. Two-Dimensional Mo1.33C MXene with Divacancy Ordering Prepared from Parent 3D Laminate with in-Plane Chemical Ordering. Nat. Commun. 2017, 8, 14949.
- Grzegórska, A.; Głuchowski, P.; Karczewski, J.; Ryl, J.; Wysocka, I.; Siuzdak, K.; Trykowski, G.; Grochowska, K.; Zielińska-Jurek, A. Enhanced Photocatalytic Activity of Accordion-like Layered Ti3C2 (MXene) Coupled with Fe-Modified Decahedral Anatase Particles Exposing and Facets. Chem. Eng. J. 2021, 426, 130801.
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An Effective 2D Light-to-Heat Conversion Material. ACS Nano 2017, 11, 3752–3759.
- Liang, J.; Chen, T.; Liu, J.; Zhang, Q.; Peng, W.C.; Li, Y.; Zhang, F.; Fan, X. Chemoselective Hydrodeoxygenation of Palmitic Acid to Diesel-like Hydrocarbons over Ni/MoO2@Mo2CTx Catalyst with Extraordinary Synergic Effect. Chem. Eng. J. 2020, 391, 123472.
- Ronda-Lloret, M.; Marakatti, V.S.; Sloof, W.G.; Delgado, J.J.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V.; Rothenberg, G.; Shiju, N.R. Butane Dry Reforming Catalyzed by Cobalt Oxide Supported on Ti2AlC MAX Phase. ChemSusChem 2020, 13, 6401–6408.
- Kurlov, A.; Deeva, E.B.; Abdala, P.M.; Lebedev, D.; Tsoukalou, A.; Comas-Vives, A.; Fedorov, A.; Müller, C.R. Exploiting Two-Dimensional Morphology of Molybdenum Oxycarbide to Enable Efficient Catalytic Dry Reforming of Methane. Nat. Commun. 2020, 11, 4920.
