In the clinical setting, the pathophysiology of sensorineural hearing loss is poorly defined and there are currently no diagnostic tests available to differentiate between subtypes. This often leaves patients with generalized treatment options such as steroids, hearing aids, or cochlear implantation. The gold standard for localizing disease is direct biopsy or imaging of the affected tissue; however, the inaccessibility and fragility of the cochlea make these techniques difficult. Thus, the establishment of an indirect biopsy, a sampling of inner fluids, is needed to advance inner ear diagnostics and allow for the development of novel therapeutics for inner ear disease. A promising source is perilymph, an inner ear liquid that bathes multiple structures critical to sound transduction. Intraoperative perilymph sampling via the round window membrane of the cochlea has been successfully used to profile the proteome, metabolome, and transcriptome of the inner ear and is a potential source of biomarker discovery. Here, we discuss the various applications of human perilymph sampling and propose a design for a sampling needle.
- perilymph
- round window
- stapedectomy
- cochlear implantation
- sensorineural hearing loss
1. Applications of Human Perilymph Sampling
1.1. Cochlear Implantation
1.2. Stapedectomy and Cochleosacculotomy
1.3. Proposed Method of RWM “Tap”
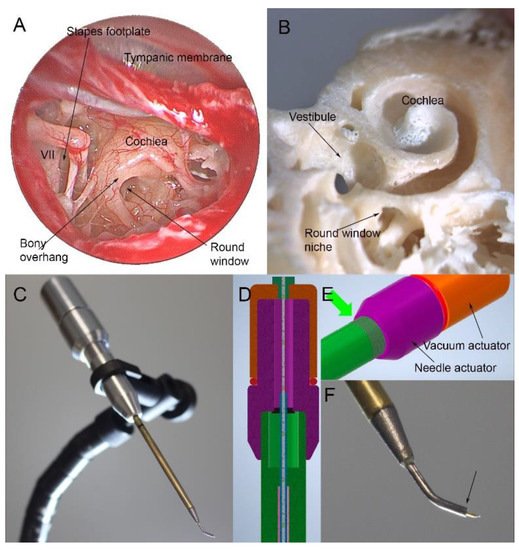
1.4. Progress in the Design of Sampling Devices
1.5. Safety and Limitations
References
- Harold C. Pillsbury; Margaret T. Dillon; Craig A. Buchman; Hinrich Staecker; Sandra M. Prentiss; Michael J. Ruckenstein; Douglas C. Bigelow; Fred F. Telischi; Diane M. Martinez; Christina Runge; et al.David R. FriedlandNikolas H. BlevinsJannine B. LarkyGeorge AlexiadesDavid M. KayliePeter S. RolandRichard T. MiyamotoDouglas D. BackousFrank M. WarrenHussam K. El-KashlanHeidi K. SlagerCarisa ReyesAllison I. RaceyOliver F. Adunka Multicenter US Clinical Trial With an Electric-Acoustic Stimulation (EAS) System in Adults: Final Outcomes. Otology & Neurotology 2018, 39, 299-305, 10.1097/mao.0000000000001691.
- Anne G.M. Schilder; Matthew P. Su; Rishi Mandavia; Caroline R. Anderson; Evie Landry; Tanjinah Ferdous; Helen Blackshaw; Early phase trials of novel hearing therapeutics: Avenues and opportunities. Hearing Research 2019, 380, 175-186, 10.1016/j.heares.2019.07.003.
- Chantal Snels; Joanna IntHout; Emmanuel Mylanus; Wendy Huinck; Ingeborg Dhooge; Hearing Preservation in Cochlear Implant Surgery: A Meta-Analysis. Otology & Neurotology 2019, 40, 145-153, 10.1097/mao.0000000000002083.
- Yvette E. Smulders; Thomas Hendriks; Robert Eikelboom; Inge Stegeman; Peter L. Santa Maria; Marcus D. Atlas; Peter L. Friedland; Predicting Sequential Cochlear Implantation Performance: A Systematic Review. Audiology and Neurotology 2016, 22, 356-363, 10.1159/000488386.
- Hideaki Moteki; Shin-Ya Nishio; Maiko Miyagawa; Keita Tsukada; Satoshi Iwasaki; Shin-Ichi Usami; Long-term results of hearing preservation cochlear implant surgery in patients with residual low frequency hearing. Acta Oto-Laryngologica 2016, 137, 516-521, 10.1080/00016489.2016.1252061.
- L. Astolfi; E. Simoni; N. Giarbini; P. Giordano; M. Pannella; S. Hatzopoulos; A. Martini; Cochlear implant and inflammation reaction: Safety study of a new steroid-eluting electrode. Hearing Research 2016, 336, 44-52, 10.1016/j.heares.2016.04.005.
- Bryan E. Pfingst; Deborah J. Colesa; Donald L. Swiderski; Aaron P. Hughes; Stefan B. Strahl; Moaz Sinan; Yehoash Raphael; Neurotrophin Gene Therapy in Deafened Ears with Cochlear Implants: Long-term Effects on Nerve Survival and Functional Measures. Journal of the Association for Research in Otolaryngology 2017, 18, 731-750, 10.1007/s10162-017-0633-9.
- Heike A. Schmitt; Andreas Pich; Anke Schröder; Verena Scheper; Giorgio Lilli; Günter Reuter; Thomas Lenarz; Proteome Analysis of Human Perilymph Using an Intraoperative Sampling Method. Journal of Proteome Research 2017, 16, 1911-1923, 10.1021/acs.jproteome.6b00986.
- Gayane Sargsyan; Natalie Kanaan; Thomas Lenarz; Anke Lesinski-Schiedat; Comparison of speech recognition in cochlear implant patients with and without residual hearing: A review of indications. Cochlear Implants International 2021, 1, 1-8, 10.1080/14670100.2021.1898111.
- Farid Alzhrani; Mohammad M. Mokhatrish; Murad O. Al-Momani; Hassan AlShehri; Abdulrahman Hagr; Soha N. Garadat; Effectiveness of stapedotomy in improving hearing sensitivity for 53 otosclerotic patients: retrospective review. Annals of Saudi Medicine 2016, 37, 49-55, 10.5144/0256-4947.2017.49.
- Pekka Persson; Henrik Harder And; Bengt Magnuson; Hearing Results in Otosclerosis Surgery after Partial Stapedectomy, Total Stapedectomy and Stapedotomy. Acta Oto-Laryngologica 1996, 117, 94-99, 10.3109/00016489709117998.
- K. Schindler; E. A. Schnieder; H. L. Wullstein; Vergleichende Bestimmung Einiger Elektrolyte und Organischer Substanzen in Der Perilymphe Otosklerosekranker Patienten. Acta Oto-Laryngologica 1964, 59, 309-319, 10.3109/00016486509124564.
- Hans P. Niedermeyer; Georg Zahneisen; Peter Luppa; Raymonde Busch; Wolfgang Arnold; Cortisol Levels in the Human Perilymph after Intravenous Administration of Prednisolone. Audiology and Neurotology 2003, 8, 316-321, 10.1159/000073516.
- John K. Niparko; Spoken Language Development in Children Following Cochlear Implantation. JAMA 2010, 303, 1498-1506, 10.1001/jama.2010.451.
- Mark J. Levenson; Rosemary B. Desloge; Simon C. Parisier; Beta-2 Transferrin. The Laryngoscope 1996, 106, 159-161, 10.1097/00005537-199602000-00010.
- Steven D. Rauch; Transferrin Microheterogeneity in Human Perilymph. The Laryngoscope 2000, 110, 545-552, 10.1097/00005537-200004000-00006.
- Giuseppe Attanasio; Marika Viccaro; Edoardo Covelli; Elio De Seta; Antonio Minni; Federica Pizzoli; Roberto Filipo; Cyclo-oxygenase enzyme in the perilymph of human inner ear. Acta Oto-Laryngologica 2010, 131, 242-246, 10.3109/00016489.2010.522593.
- Ottó Ribári; István Sziklai; Cathepsin D Activity in Otosclerotic Bone and Perilymph. Acta Oto-Laryngologica 1987, 105, 549-552, 10.3109/00016488809119518.
- Causse, J.R.; Uriel, J.; Berges, J.; Shambaugh, G.E.; Bretlau, P.; Causse, J.B; The enzymatic mechanism of the . Am. J. Otol 1982, 3, 297-314.
- J. H. Fritsch; C. R. Jolliff; XC Protein Components of Human Perilymph. Annals of Otology, Rhinology & Laryngology 1966, 75, 1070-1076, 10.1177/000348946607500416.
- R. Hladk; Z. Brada; A. Kočent; Versuch Einer Biochemischen Biopsie Der Perilymphe Bei Operierten Kranken. Acta Oto-Laryngologica 1959, 51, 424-428, 10.3109/00016486009124515.
- Jacob, M.; Causse, J.; Gaudy, D.; Duru, C.; Causse, J.B.; Puech, A.; Antibacterial therapy in surgery of the inner and middle ear. A study of co-trimoxazole penetration into the perilymph . Nouv. Presse Med 1982, 11, 2205-2209.
- L. Rüedi; M. C. Sanz; U. Fisch; Untersuchung Der Perilymphe Nach Stapedktomie in Otosklerosefàallen. Acta Oto-Laryngologica 1964, 59, 289-308, 10.3109/00016486509124563.
- Franz Altmann; Mario Kornfeld; John J. Shea; I Inner Ear Changes in Otosclerosis. Annals of Otology, Rhinology & Laryngology 1966, 75, 5-32, 10.1177/000348946607500101.
- L.-G. Chevance; J. R. Causse; 1-Antitrypsin Activity of Perilymph: Occurrence During Progression of Otospongiosis. Archives of Otolaryngology - Head and Neck Surgery 1976, 102, 363-364, 10.1001/archotol.1976.00780110075008.
- Matthew Shew; Athanasia Warnecke; Thomas Lenarz; Heike Schmitt; Sumedha Gunewardena; Hinrich Staecker; Feasibility of microRNA profiling in human inner ear perilymph. NeuroReport 2018, 29, 894-901, 10.1097/wnr.0000000000001049.
- William C. Kinney; Nancy Nalepa; Gordon B. Hughes; Sam E. Kinney; Cochleosacculotomy for the treatment of meniere's disease in the elderly patient. The Laryngoscope 1995, 105, 934-937, 10.1288/00005537-199509000-00012.
- Takeshi Fujita; Jung Eun Shin; Marybeth Cunnane; Kyoko Fujita; Simon Henein; Demetri Psaltis; Konstantina M. Stankovic; Surgical Anatomy of the Human Round Window Region. Otology & Neurotology 2016, 37, 1189-1194, 10.1097/mao.0000000000001074.
- Hsiao-Chun Lin; Yin Ren; Andrew C. Lysaght; Shyan-Yuan Kao; Konstantina M. Stankovic; Proteome of normal human perilymph and perilymph from people with disabling vertigo. PLOS ONE 2019, 14, e0218292, 10.1371/journal.pone.0218292.
- Andrew C. Lysaght; Shyan-Yuan Kao; Joao A. Paulo; Saumil N. Merchant; Hanno Steen; Konstantina M. Stankovic; Proteome of Human Perilymph. Journal of Proteome Research 2011, 10, 3845-3851, 10.1021/pr200346q.
- Kyu Yup Lee; Takayuki Nakagawa; Takayuki Okano; Ryusuke Hori; Kazuya Ono; Yasuhiko Tabata; Sang Heun Lee; Juichi Ito; Novel Therapy for Hearing Loss. Otology & Neurotology 2007, 28, 976-981, 10.1097/mao.0b013e31811f40db.
- Matthew Shew; Helena Wichova; Andres Bur; Devin C. Koestler; Madeleine St Peter; Athanasia Warnecke; Hinrich Staecker; MicroRNA Profiling as a Methodology to Diagnose Ménière’s Disease: Potential Application of Machine Learning. Otolaryngology–Head and Neck Surgery 2020, 164, 399-406, 10.1177/0194599820940649.
- Stefan K. Plontke; Jared J. Hartsock; Ruth M. Gill; Alec N. Salt; Intracochlear Drug Injections through the Round Window Membrane: Measures to Improve Drug Retention.. Audiology and Neurotology 2016, 21, 72-9, 10.1159/000442514.
- Aykut Aksit; Daniel N. Arteaga; Miguel Arriaga; Xun Wang; Hirobumi Watanabe; Karen Kasza; Anil K. Lalwani; Jeffrey W. Kysar; In-vitro perforation of the round window membrane via direct 3-D printed microneedles. Biomedical Microdevices 2018, 20, 1-12, 10.1007/s10544-018-0287-3.
- Harry Chiang; Michelle Yu; Aykut Aksit; Wenbin Wang; Sagit Stern-Shavit; Jeffrey W. Kysar; Anil K. Lalwani; 3D-Printed Microneedles Create Precise Perforations in Human Round Window Membrane in Situ. Otology & Neurotology 2020, 41, 277-284, 10.1097/mao.0000000000002480.
- Hirobumi Watanabe; Luis Cardoso; Anil K. Lalwani; Jeffrey W. Kysar; A dual wedge microneedle for sampling of perilymph solution via round window membrane. Biomedical Microdevices 2016, 18, 1-8, 10.1007/s10544-016-0046-2.
- Betsy Szeto; Aykut Aksit; Chris Valentini; Michelle Yu; Emily G. Werth; Shahar Goeta; Chuanning Tang; Lewis M. Brown; Elizabeth S. Olson; Jeffrey W. Kysar; et al.Anil K. Lalwani Novel 3D-printed hollow microneedles facilitate safe, reliable, and informative sampling of perilymph from guinea pigs. Hearing Research 2020, 400, 108141, 10.1016/j.heares.2020.108141.
- Samuel Early; In Seok Moon; Krishna Bommakanti; Ian Hunter; Konstantina M. Stankovic; A novel microneedle device for controlled and reliable liquid biopsy of the human inner ear. Hearing Research 2019, 381, 107761, 10.1016/j.heares.2019.06.004.
- William H. Lippy; Leonard P. Berenholz; Revision Stapedectomy. Ear, Nose & Throat Journal 2009, 88, 1260-1260, 10.1177/014556130908801207.
- Wolfgang Gstoettner; Silke Helbig; Claudia Settevendemie; Uwe Baumann; Jens Wagenblast; Christoph Arnoldner; A new electrode for residual hearing preservation in cochlear implantation: first clinical results. Acta Oto-Laryngologica 2008, 129, 372-379, 10.1080/00016480802552568.
- Oliver F. Adunka; Stefan Mlot; Thomas A. Suberman; Adam P. Campbell; Joshua Surowitz; Craig A. Buchman; Douglas C. Fitzpatrick; Intracochlear Recordings of Electrophysiological Parameters Indicating Cochlear Damage. Otology & Neurotology 2010, 31, 1233-1241, 10.1097/mao.0b013e3181f1ffdf.
- Alec N. Salt; Christian Kellner; Shane Hale; Contamination of perilymph sampled from the basal cochlear turn with cerebrospinal fluid. Hearing Research 2003, 182, 24-33, 10.1016/s0378-5955(03)00137-0.
- Giovanni Bianchin; Valeria Polizzi; Patrizia Formigoni; Carmela Russo; Lorenzo Tribi; Cerebrospinal Fluid Leak in Cochlear Implantation: Enlarged Cochlear versus Enlarged Vestibular Aqueduct (Common Cavity Excluded). International Journal of Otolaryngology 2016, 2016, 1-9, 10.1155/2016/6591684.
- Liu Hongjian; Wang Guangke; Ma Song; Ding Xiaoli; Zhang Daoxing; The prediction of CSF gusher in cochlear implants with inner ear abnormality. Acta Oto-Laryngologica 2012, 132, 1271-1274, 10.3109/00016489.2012.701328.
- H. J. Yi; Wei Guo; N. Wu; J. N. Li; H. Z. Liu; L. L. Ren; P. N. Liu; S. M. Yang; The temporal bone microdissection of miniature pigs as a useful large animal model for otologic research. Acta Oto-Laryngologica 2013, 134, 26-33, 10.3109/00016489.2013.835866.
