Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Alice Luddi.
Taste receptors were first described as sensory receptors located on the tongue, where they are expressed in small clusters of specialized epithelial cells. Taste receptors and components of the coupled taste transduction cascade are also expressed during the different phases of spermatogenesis as well as in mature spermatozoa from mouse to humans and the overlap between the ligand spectrum of taste receptors with compounds in the male and female reproductive organs makes it reasonable to assume that sperm “taste” these different cues in their natural microenvironments.
- sperm
- taste receptor
1. Taste Receptors and Signal Transduction
The name “Taste receptors” (TAS) derives from their first identification in the oral cavity [1] and their role in the sensation of gustation. Indeed, they were first classified as sensory receptors, whose expression was limited to small clusters of specialized epithelial cells which reside within taste buds located on the tongue [2].
The sensation of taste can be divided into five distinct categories [3]: (i) sweet, for detection of sugars and sweeteners; (ii) salty, for detection of sodium; (iii) umami, for detection of all L-amino acids in rodents [4] but only of L-glutamate in humans [5], required by the body for energy balance and building proteins; (iv) sour, which perceives acids in unripe fruit and spoiled foods and (v) bitter, which detects a variety of alkaloid substances, many of which are toxic. However, taste receptors for non-canonical taste stimuli have been described; among them are receptors for kokumi, a stimulus that enhances the basic taste sensations [6] and fatty acid transporters (receptor for fat), involved in oral detection of different fatty acids [7]. Taste sense acts as a guardian and guides for our eating habits: The sensations of bitter and/or sour act as a deterrent ingesting toxic substances and strong acids, while the sensations of sweet, umami and salty lead us to prefer foods containing carbohydrates, amino acids and sodium [8]. Consequently, it is not surprising that the capability to detect and react to chemical stimuli is a trait possessed by the simplest forms of life [9].
Taste transduction signaling involves the interaction of molecules (i.e., tastants) with their specific taste receptors, expressed by cells residing in the taste buds. Taste buds are the transducing endorgans of gustation and each bud comprises 50–100 elongated cells located on the connective papillae of the tongue and scattered throughout the epithelium of the soft palate and larynx. Taste buds are onion-shaped structures. They extend from the basal lamina to the surface of the tongue, where their apical microvilli encounter taste stimuli in the oral cavity, detecting and distinguishing between bitter, sweet, sour, salty and umami stimuli.
Salts and acids utilize apically located ion channels for transduction, while bitter, sweet and umami (L-glutamate) utilize G protein-coupled receptors (GPCRs) and a subsequent second-messenger signal transduction process (Figure 1). If compared with other GPCRs, TAS are low-affinity receptors, with binding affinities in the micro- to the millimolar range, typical for the concentration of most nutrients in foods [10].
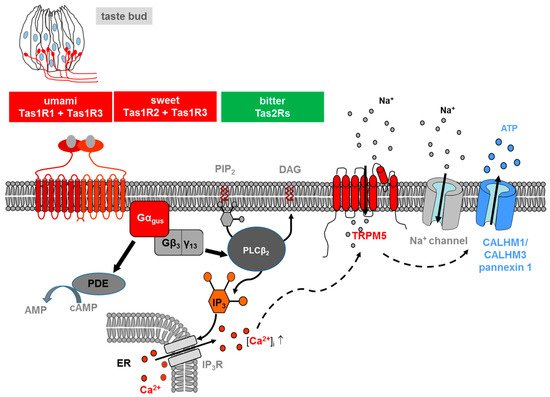
Figure 1. Transduction of L-glutamate (umami), sweet and bitter stimuli in taste receptor cells on the tongue. Ligand-induced stimulation of the umami (Tas1r1+Tas1r3), sweet (Tas1r2+Tas1r3) or bitter receptors (Tas2rs) expressed at the apical membrane of type II taste cells within a taste bud (s. drawing in the left) activates in all cases a trimeric G protein composed of α-gustducin (Gαgus) and a complex consisting ofGβ3 andGγ13 (Gβ3/γ13). The released Gβγ-complex activates phospholipase C isoform β2 (PLCβ2) which then induces production of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG); the second messenger IP3, in turn, activates the IP3 receptor (IP3R), an intracellular ion channel that allows Ca2+ release from the intracellular endoplasmic reticulum (ER) store (solid lines). An increase in intracellular Ca2+ then activates the transient receptor potential melastatin 5 (TRPM5), a plasma membrane-localized sodium-selective channel which leads to depolarization and subsequent activation of voltage-gated sodium channels (Na+ channel) (dashed lines). The combined action of elevated Ca2+ and membrane depolarization opens the calcium homeostasis modulator (CALHM) channel, composed of CALHM1 and CALHM3 and pannexin1 channels, thus resulting in the release of the neurotransmitter ATP. At the same time, α-gustducin activates a phosphodiesterase (PDE) (solid lines), which catalyzes the hydrolysis of the second messenger cyclic-AMP (cAMP) to AMP. For the sake of simplicity, the regulatory effects of cAMP are omitted in the model.
Two different families of taste GPCRs have been identified: Type 1 Taste Receptors (Tas1s) and Type 2 Taste Receptors (Tas2s): Tas1s encode the receptor proteins for sweet and umami taste, while Tas2s mediate bitter taste [11,12][11][12].
Three different Tas1s have been identified, which are products of the Tas1s genes: TAS1R1, TAS1R2 and TAS1R3 [11,13][11][13]. These receptors are activated only if assembled into heterodimers: TAS1R3 heterodimerizes with TAS1R1, thereby forming the umami receptor (TAS1R1 + TAS1R3); assembly of TAS1R3 with TAS1R2 led to the formation of a sweet receptor (TAS1R2 + TAS1R3), activated by carbohydrates, artificial sweeteners and sweet proteins [14,15][14][15]. TAS1R3 may also serve as a low-affinity sweet receptor alone, perhaps as a homodimer or homomultimer [16]. The taste 2 receptors, consisting of a large family including about 25 different isoforms in humans and about 35 in rodents, are responsible for the sensation of bitter tastants [12,17,18][12][17][18].
The signaling of both TAS1Rs and TAS2Rs is mediated by the same intracellular transduction pathway in type II taste bud cells [10,19,20][10][19][20] (Figure 1). The binding of the corresponding ligand activates a heterotrimeric G protein, which consists in most cells of the G protein α-gustducin and β3/γ13, leading to the release of the G β/γ subunits and subsequent stimulation of phospholipase C isoform β2 (PLCβ2), which, in turn, hydrolyses the membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2) to produce the two-second messengers inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG) [8,10,20][8][10][20]. IP3 opens IP3 receptor (IP3R3) type 3 ion channels on the endoplasmic reticulum membrane, thus releasing calcium (Ca2+) into the cytosol of the activated receptor cell. As a result, an increase in the intracellular Ca2+ level activates the cation channel transient receptor potential, melastatin family member 5 (TRPM5) [21,22][21][22]. The TRPM5-triggered influx of Na+ and activation of voltage-dependent sodium channels, subsequently depolarize the cell, leading to a release of the neurotransmitter adenosine triphosphate (ATP) through pannexin 1 and a hexameric channel composed of Calcium homeostasis modulator (CALHM) 1 and CALHM3 [23,24,25][23][24][25]. ATP finally transmits the signal to ionotropic purinergic receptors P2X2 and P2X3 receptors on gustatory afferent fibers [26,27][26][27]. Simultaneously released α-gustducin activates phosphodiesterase, thus resulting in a decrease of intracellular levels of the second messenger cyclic adenosine monophosphate (cAMP) [28].
2. Taste Receptors and Spermatogenesis
2.1. Spermatogenesis
Spermatogenesis is a complex and precisely controlled cellular transformation process that ensures the production of millions of sperm daily [53][29]. This massive sperm production takes place in the tightly packed seminiferous tubules of the two testes where each tubular unit contains distinct concentric layers of germ cells of different stages of maturation (Figure 2): Diploid spermatogonia, the stem cells of the testis, are localized in the basal cell layer of the seminiferous tubules. Upon mitotic divisions that provide the necessary cell number essential for a high sperm output, developing spermatocytes move to the more luminal part of the seminiferous tubule where they undergo meiosis resulting in the generation of haploid spermatids. The round spermatids subsequently run through a cellular transformation process called spermiogenesis in which they differentiate into spermatozoa finally localized into the luminal region of the tubular unit [54][30].
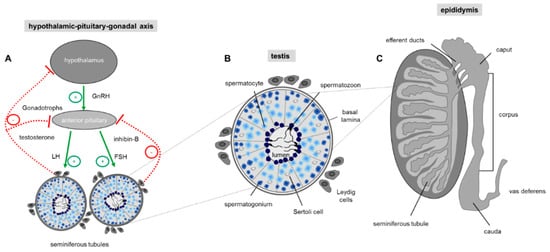

Figure 2. Regulation of sperm production. (A) Hormonal control of spermatogenesis in the testis. Spermatogenesis in the testis is under endocrine and paracrine control, which is regulated by the hypothalamus and the pituitary gland also known as the hypothalamic-pituitary-gonadal (HPG) axis. The hypothalamus regulates the hormonal activity of the anterior pituitary gland by secreting the tropic gonadotropin-releasing hormone (GnRH). Upon binding of GnRH to the anterior pituitary gland production of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) is elevated which upon bloodstream transport stimulate testosterone secretion by intestinal Leydig cells and activation of Sertoli cells by FSH. Sertoli cells as a cellular part of the tubular unit provide the optimal environment for the developing germ cells. Negative feedback of GnRH production in the hypothalamic neurons and LH/FSH secretion by the pituitary gland is exerted by high testosterone levels in the blood and secretion of the proteohormone inhibin-B by Sertoli cells. Arrow: positive (green) and negative (red) feedback. (B) Schematic drawing of a single seminiferous tubule with different stages of developing germ cells during spermatogenesis. The cross-section shows that germ cells of a distinct developmental stage are organized in concentric layers within the tubule: In the most basal cell layer of the tubular unit, the immature spermatogonial stem cells are located, followed by spermatocytes, round spermatids and finally the most mature elongated spermatids which are concentrated in the lumen of the seminiferous tubule. The regulation of spermatogenesis is mainly mediated by surrounding interstitial Leydig cells which produce testosterone. The Sertoli cells within the seminiferous tubules have a nurturing role for the developing germ cell and transduce the action of FSH to the closely associated germ cells. (C) Schematic drawing showing a sagittal section through a whole testis and the overlying epididymis. The testis contains the tightly packed seminiferous tubules where spermatogenesis takes place. The elongated duct presenting the epididymis at the posterior margin of the testis is subdivided into three discrete segments (caput, corpus, cauda), where the luminal fluid of each region is characterized by a unique composition of different constituents, essential for post-testicular sperm maturation.
Continuous sperm production in adult males depends on endocrine and testicular paracrine/autocrine factors which together coordinate proliferation and germ cell differentiation [55,56][31][32]. The endocrine stimulation of spermatogenesis involves the two gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Their secretion by the anterior pituitary gland is controlled through the hypothalamic-pituitary portal system with gonadotropin-releasing hormone (GnRH) secreted by the hypothalamus [55][31] (Figure 2A). The subsequent action of the two glycoproteins LH and FSH require cell to cell communication within the testis which is predominantly mediated by the two somatic cell types within the testis, the Leydig and Sertoli cells. Leydig cells, that reside between the seminiferous tubules of the testis, produce testosterone upon LH stimulation [57][33]. Sertoli cells, which form cytoplasmic bridges with the developing germ cells within the seminiferous tubules, are the ‘nurse’ cell of the testes [58][34] and play a more comprehensive role: Sertoli cells create an adequate ionic environment for germ cell development, have a nurturing role for differentiating sperm, phagocytose residual bodies after spermiogenesis and assist in the final migration of mature spermatozoa into the lumen of the seminiferous tubule [59][35]. In addition, since the germ cells do not possess receptors for FSH and testosterone, Sertoli cells represent the major cellular targets for hormonal signaling so that the effect of hormones on germ cell development is indirect [60][36]. The hypothalamic-pituitary-gonadal (HPG) axis is a self-regulating system with two negative feedback loops (Figure 2A): on one hand, high testosterone concentrations in the peripheral blood provide a negative feedback route to suppress hypothalamic discharge of GnRH and consequently LH release from the anterior pituitary [61][37]. The second loop is the release of inhibin-B by Sertoli cells. Inhibin-B has a negative feedback effect on the pituitary gland, thereby suppressing FSH secretion [62][38] (Figure 2A).
2.2. Apoptosis
However, the success of germ cell proliferation and differentiation is also ensured by a dynamic balance between germ cell development and a carefully controlled process of programmed cell death, thereby ensuring a selective elimination of an overrun of produced germ cells and in addition deletion of abnormal and defective sperm [63][39]. Removal of an excess of germ cells taking place during spermatogenesis in the testis and ensuring an optimal ratio of supporting Sertoli cells to germ cells during at all stages of development leads to a degeneration of about 75% of spermatogonia before reaching maturity [64,65][40][41]. In maturated and ejaculated sperm where apoptosis also occurs [66][42] the process of programmed cell death is responsible to eliminate damaged cells [64][40]. Any imbalance in the apoptotic process has dramatic implications for male infertility: whereas a decrease in the selective elimination of defective developing and mature sperm causes poor sperm quality, an increase in apoptosis could potentially lead to a reduced sperm count and thus sub-fertility [67,68][43][44]. Thus, identification of “death triggering signals” [69][45] as well as corresponding receptor proteins that elicit activation of the apoptotic machinery is of critical importance for the fertilization potential of males. Although not fully understood [64][40], the onset of apoptosis in germ cells can not only be induced by the lack of hormones, like gonadotropins and testosterone [56][32] but also by a broad range of non-hormonal and also non-physiological stimuli, such as heat stress, industrial and therapeutic agents as well as a variety of naturally occurring toxicants [70,71][46][47]. In this context, one has to consider that receptors belonging to the taste 2 family are specialized to detect bitter compounds including extremely toxic alkaloids [72,73][48][49]. Since genes for all 35 bitter receptors have been identified in mouse testis [74][50] bitter receptors might present promising candidates to detect testicular toxicants. Moreover, genetic deletion of the Tas1r1 receptor, the dimerization partner of the Tas1r3 which in taste buds on the tongue forms the functional receptor for L-glutamate (umami), leads to a significant increase in the number of apoptotic cells during spermatogenesis [42][51], an observation that already indicates that taste receptors indeed play a functional role for controlling apoptosis in the male reproductive tissue.
References
- Hoon, M.A.; Adler, E.; Lindemeier, J.; Battey, J.F.; Ryba, N.J.; Zuker, C.S. Putative mammalian taste receptors: A class of taste-specific GPCRs with distinct topographic selectivity. Cell 1999, 96, 541–551.
- Roper, S.D.; Chaudhari, N. Taste buds: Cells, signals and synapses. Nat. Rev. Neurosci. 2017, 18, 485–497.
- Lindemann, B. Receptors and transduction in taste. Nature 2001, 413, 219–225.
- Nelson, G.; Chandrashekar, J.; Hoon, M.A.; Feng, L.; Zhao, G.; Ryba, N.J.P.; Zuker, C.S. An amino-acid taste receptor. Nature 2002, 416, 199.
- Li, F.; Zhou, M. Depletion of bitter taste transduction leads to massive spermatid loss in transgenic mice. Mol. Hum. Reprod. 2012, 18, 289–297.
- Kuroda, M.; Miyamura, N. Mechanism of the perception of “kokumi” substances and the sensory characteristics of the “kokumi” peptide, γ-Glu-Val-Gly. Flavour 2015, 4, 11.
- Besnard, P.; Passilly-Degrace, P.; Khan, N.A. Taste of Fat: A Sixth Taste Modality? Physiol. Rev. 2016, 96, 151–176.
- Finger, T.E.; Kinnamon, S.C. Taste isn’t just for taste buds anymore. F1000 Biol. Rep. 2011, 3, 20.
- Strosberg, A.D. Structure/function relationship of proteins belonging to the family of receptors coupled to GTP-binding proteins. Eur. J. Biochem. 1991, 196, 1–10.
- Kinnamon, S.C. Taste receptor signalling—From tongues to lungs. Acta Physiol. 2012, 204, 158–168.
- Bachmanov, A.A.; Beauchamp, G.K. Taste receptor genes. Annu. Rev. Nutr. 2007, 27, 389–414.
- Chandrashekar, J.; Hoon, M.A.; Ryba, N.J.P.; Zuker, C.S. The receptors and cells for mammalian taste. Nature 2006, 444, 288–294.
- Montmayeur, J.P.; Matsunami, H. Receptors for bitter and sweet taste. Curr. Opin. Neurobiol. 2002, 12, 366–371.
- Kim, S.-K.; Chen, Y.; Abrol, R.; Goddard, W.A.; Guthrie, B. Activation mechanism of the G protein-coupled sweet receptor heterodimer with sweeteners and allosteric agonists. Proc. Natl. Acad. Sci. USA 2017, 114, 2568–2573.
- Cui, M.; Jiang, P.; Maillet, E.; Max, M.; Margolskee, R.F.; Osman, R. The heterodimeric sweet taste receptor has multiple potential ligand binding sites. Curr. Pharm. Des. 2006, 12, 4591–4600.
- Treesukosol, Y.; Smith, K.R.; Spector, A.C. The functional role of the T1R family of receptors in sweet taste and feeding. Physiol. Behav. 2011, 105, 14–26.
- Margolskee, R.F.; Dyer, J.; Kokrashvili, Z.; Salmon, K.S.H.; Ilegems, E.; Daly, K.; Maillet, E.L.; Ninomiya, Y.; Mosinger, B.; Shirazi-Beechey, S.P. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl. Acad. Sci. USA 2007, 104, 15075–15080.
- Meyerhof, W.; Born, S.; Brockhoff, A.; Behrens, M. Molecular biology of mammalian bitter taste receptors. A review. Flavour Fragr. J. 2011, 26, 260–268.
- Chaudhari, N.; Roper, S.D. The cell biology of taste. J. Cell Biol. 2010, 190, 285–296.
- Liman, E.R.; Zhang, Y.V.; Montell, C. Peripheral Coding of Taste. Neuron 2014, 81, 984–1000.
- Liu, D.; Liman, E.R. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl. Acad. Sci. USA 2003, 100, 15160–15165.
- Hofmann, T.; Chubanov, V.; Gudermann, T.; Montell, C. TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr. Biol. 2003, 13, 1153–1158.
- Taruno, A.; Matsumoto, I.; Ma, Z.; Marambaud, P.; Foskett, J.K. How do taste cells lacking synapses mediate neurotransmission? CALHM1, a voltage-gated ATP channel. Bioessays 2013, 35, 1111–1118.
- Huang, Y.-J.; Maruyama, Y.; Dvoryanchikov, G.; Pereira, E.; Chaudhari, N.; Roper, S.D. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc. Natl. Acad. Sci. USA 2007, 104, 6436–6441.
- Ma, Z.; Taruno, A.; Ohmoto, M.; Jyotaki, M.; Lim, J.C.; Miyazaki, H.; Niisato, N.; Marunaka, Y.; Lee, R.J.; Hoff, H.; et al. CALHM3 Is Essential for Rapid Ion Channel-Mediated Purinergic Neurotransmission of GPCR-Mediated Tastes. Neuron 2018, 98, 547–561.
- Huang, Y.A.; Stone, L.M.; Pereira, E.; Yang, R.; Kinnamon, J.C.; Dvoryanchikov, G.; Chaudhari, N.; Finger, T.E.; Kinnamon, S.C.; Roper, S.D. Knocking out P2X receptors reduces transmitter secretion in taste buds. J. Neurosci. 2011, 31, 13654–13661.
- Finger, T.E.; Danilova, V.; Barrows, J.; Bartel, D.L.; Vigers, A.J.; Stone, L.; Hellekant, G.; Kinnamon, S.C. ATP Signaling Is Crucial for Communication from Taste Buds to Gustatory Nerves. Science 2005, 310, 1495–1499.
- Clapp, T.R.; Trubey, K.R.; Vandenbeuch, A.; Stone, L.M.; Margolskee, R.F.; Chaudhari, N.; Kinnamon, S.C. Tonic activity of Galpha-gustducin regulates taste cell responsivity. FEBS Lett. 2008, 582, 3783–3787.
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17.
- Bergmann, M. Spermatogenesis—Physiology and pathophysiology. Urol. A 2005, 44, 44.
- Choi, D. The consequences of mutations in the reproductive endocrine system. Dev. Reprod. 2012, 16, 235–251.
- O’Shaughnessy, P.J. Hormonal control of germ cell development and spermatogenesis. Semin. Cell Dev. Biol. 2014.
- Ramaswamy, S.; Weinbauer, G.F. Endocrine control of spermatogenesis: Role of FSH and LH/ testosterone. Spermatogenesis 2014, 4, e996025.
- Crisóstomo, L.; Alves, M.G.; Gorga, A.; Sousa, M.; Riera, M.F.; Galardo, M.N.; Meroni, S.B.; Oliveira, P.F. Molecular mechanisms and signaling pathways involved in the nutritional support of spermatogenesis by sertoli cells. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; ISBN 978-1-49-397698-0.
- Alves, M.G.; Rato, L.; Carvalho, R.A.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell. Mol. Life Sci. 2013, 70, 777–793.
- De Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13, 1–18.
- Ubuka, T.; Son, Y.L.; Tobari, Y.; Narihiro, M.; Bentley, G.E.; Kriegsfeld, L.J.; Tsutsui, K. Central and direct regulation of testicular activity by gonadotropin-inhibitory hormone and its receptor. Front. Endocrinol. 2014, 5, 8.
- Barakat, B.; Itman, C.; Mendis, S.H.; Loveland, K.L. Activins and inhibins in mammalian testis development: New models, new insights. Mol. Cell. Endocrinol. 2012, 359, 66–77.
- Xu, Y.R.; Dong, H.S.; Yang, W.X. Regulators in the apoptotic pathway during spermatogenesis: Killers or guards? Gene 2016, 582, 97–111.
- Aitken, R.J.; Baker, M.A. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int. J. Dev. Biol. 2013, 57, 265–272.
- Boekelheide, K.; Fleming, S.L.; Johnson, K.J.; Patel, S.R.; Schoenfeld, H.A. Role of Sertoli cells in injury-associated testicular germ cell apoptosis (44558). Proc. Soc. Exp. Biol. Med. 2000, 225, 105–115.
- Grunewald, S.; Sharma, R.; Paasch, U.; Glander, H.J.; Agarwal, A. Impact of caspase activation in human spermatozoa. Microsc. Res. Tech. 2009, 72, 878–888.
- Shaha, C. Modulators of spermatogenic cell survival. Soc. Reprod. Fertil. Suppl. 2007, 63, 173–186.
- Shukla, K.K. Apoptosis, spermatogenesis and male infertility. Front. Biosci. 2012, 4, 746–754.
- Sinha Hikim, A. Hormonal and genetic control of germ cell apoptosis in the testis. Rev. Reprod. 1999, 4, 38–47.
- Mima, M.; Greenwald, D.; Ohlander, S. Environmental Toxins and Male Fertility. Curr. Urol. Rep. 2018, 19, 50.
- Mathur, P.P.; D’Cruz, S.C. The effect of environmental contaminants on testicular function. Asian J. Androl. 2011, 13, 585.
- Jaggupilli, A.; Howard, R.; Upadhyaya, J.D.; Bhullar, R.P.; Chelikani, P. Bitter taste receptors: Novel insights into the biochemistry and pharmacology. Int. J. Biochem. Cell Biol. 2016, 77, 184–196.
- Behrens, M.; Meyerhof, W. Vertebrate Bitter Taste Receptors: Keys for Survival in Changing Environments. J. Agric. Food Chem. 2018, 66, 2204–2213.
- Xu, J.; Cao, J.; Iguchi, N.; Riethmacher, D.; Huang, L. Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol. Hum. Reprod. 2013, 19, 17–28.
- Meyer, D.; Voigt, A.; Widmayer, P.; Borth, H.; Huebner, S.; Breit, A.; Marschall, S.; de Angelis, M.H.; Boehm, U.; Meyerhof, W.; et al. Expression of tas1 taste receptors in mammalian spermatozoa: Functional role of tas1r1 in regulating basal ca2+and camp concentrations in spermatozoa. PLoS ONE 2012, 7, e32354.
More
