Supercritical water gasification (SCWG) is a promising technology for the valorization of
wet biomass with a high-water content, which has attracted increasing interest. Many experimental
studies have been carried out using conventional heating equipment at lab scale, where researchers
try to obtain insight into the process. However, heat transfer from the energy source to the fluid
stream entering the reactor may be ineffective, so slow heating occurs that produces a series of
undesirable reactions, especially char formation and tar formation. This paper reviews the limitations
due to different factors affecting heat transfer, such as low Reynolds numbers or laminar flow regimes,
unknown real fluid temperature as this is usually measured on the tubing surface, the strong change
in physical properties of water from subcritical to supercritical that boosts a deterioration in heat
transfer, and the insufficient mixing, among others. In addition, some troubleshooting and new
perspectives in the design of efficient and effective devices are described and proposed to enhance
heat transfer, which is an essential aspect in the experimental studies of SCWG to move it forward to
a larger scale.
- supercritical water
- gasification
- reforming
- heat transfer
- tubular reactor
- heating rate
1. Introduction
2. Heat Transfer Deterioration in SCWG Experiments
The abrupt changes that water undergoes in its physical properties near the critical point contribute to the deterioration of heat transfer, due to the decrease in turbulent thermal conductivity and eddy viscosity, leading to lower turbulent heat flux and therefore higher surface temperatures [39]. This deterioration may be the result of a significant density variation near the critical point that deforms the velocity profile, flagging the turbulence [5], or even due to the thickening of the viscous underlayer depending on the fluid flow rate [40]. Many research works, mainly performed numerically, have been published on this phenomenon, known as heat transfer deterioration (HTD) [41,42,43,44,45,46,47][41][42][43][44][45][46][47]. Recently, Wang et al. [48] obtained, by simulation, that semicircular heating decreased the degree of HTD due to the greater turbulence produced by the greater density difference between the heating side and the adiabatic side. Figure 1 illustrates the trend of the physical properties of water versus temperature at 23 MPa and 25 MPa, obtained by [21], where the maximum values of specific heat and thermal conductivity define different pseudocritical temperatures; in fact, each pair (P, T) above the critical point leads to a pseudocritical point. The pseudocritical temperature increases as the (supercritical) pressure increases, and at this point and on, there is no longer a difference between the liquid and gas/vapor phases.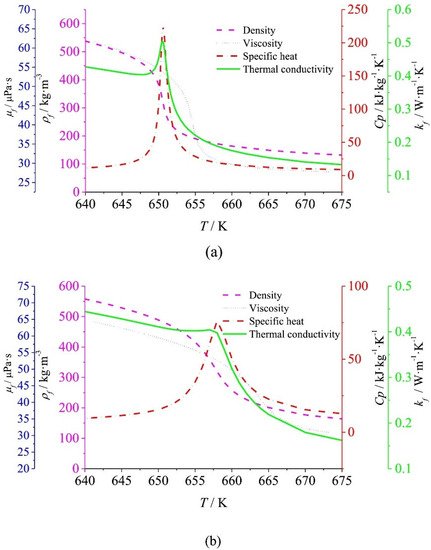
3. Next Perspectives in Design
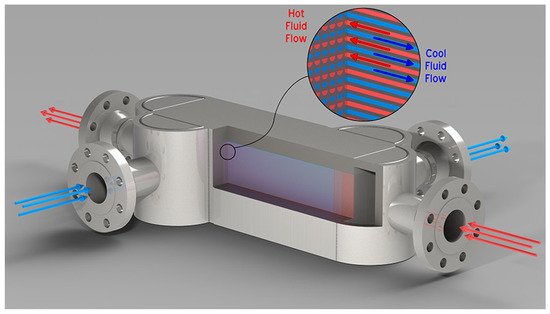
On the basis of the concept of a tubular reactor that may be extended to a multitube reactor, some ideas may be proposed and discussed to enhance heat transfer by designing involved process units. There must be fast and effective heating inside the reactor, where fluid flows, and that is the zone where the highest resistance to heat transfer is found. In this way, a new reactor design could enhance this aspect. Here, process intensification may play an important role and there are a number of possibilities. First, a nonmetallic reactor made of quartz could be used, for instance, instead of metallic alloys, such as Inconel 600 or 625, AISI 316, or different Hastelloy, along with microwave heating, where the frequency would be adjusted to selectively supply the energy required by the reactants to allow their molecules to overcome the activation energy, which is the energy barrier. This could be more efficient for the energy supply required for the reactions and even for the heating of all of the streams, which would take place more quickly and selectively. In fact, considering an Arrhenius-type kinetic law, heating must serve to increase the temperature term found in the exponential function. This heating way, against electric heating by resistances (directly disposed on the tube wall or applied externally to the reactor and transmitted by radiation), avoids temperature gradients that produce an energy distribution, and hence energy losses. Second, a maximum specific surface area should be used to further improve mass and heat transfer. Thus, different and specific heat transfer equipment and reactors may be applied to achieve this target. The diffusion-bonded compact heat exchanger is a compact type heat changer that can operate up to 100 MPa or 900 °C, reducing up to 90% of the area compared to multitube heat exchangers, where the heat transfer area per unit volume is at least 1000 m2/m3, so the size can be reduced to 85% compared to conventional multitube heat transfer [77][54]. This heat exchanger could be used as both a microreactor and a microheat exchanger. Mortean et al. [78][55] evaluated heat transfer in a diffusion-bonded compact heat exchanger at low flow rates for low Reynolds numbers, mainly in the laminar flow regime, and more recently Sarmiento et al. [79][56] extended the study in a series of Reynolds numbers between 2600 and 7500, thus covering the transition to a turbulent flow regime. Currently, there are already several manufacturers [80[57][58],81], and Figure 72 shows the details of one of them. Likewise, microchannel reactors show a surface-to-volume ratio much larger than that of multitube reactors, which could be the logical selection for scaling up as tubes may be multiplied as the flow rate does, especially considering that the lower thickness of small ID tubes has mechanical resistance similar to that of tubes with larger ID and thickness under the severe conditions of SWG. The use of microreactors with thousands of microchannels allows for good mixing of reactants and the removal of heat is more efficient. There is another possibility of a micro reactor plus a micro heat exchanger. Regarding heat transfer in lab-scale tests, apart from using a different energy source, the use of compact devices, such as those mentioned above, could allow for better heat transfer performance using flue gas coming from a burner at high temperature acting as the hot fluid. In addition, the design of a series of heat exchangers should be performed, especially the one where the transition from subcritical to supercritical state takes place because of the strong change in the physical properties of the feed, mainly wet biomass with a high proportion of water. Furthermore, the use of static mixers promotes turbulence without mobile parts and presents resistance to interphase mass transfer that is noticeably smaller than in conventional equipment, such as stirred tanks, and the flow pattern is close to the plug flow and is more uniform, and the residence time of the molecules is closely distributed. As a consequence, the collisions of molecules will be more effective and energy losses will be lessened [82][59]. It is clear that despite the proposed devices being real, further research must be carried out to improve their performance and applicability and, in particular, to reduce capital costs so that they may be used at full scale. (see references in the original paper published in Energies)References
- Savage, P.E. Organic Chemical Reactions in Supercritical Water. Chem. Rev. 1999, 99, 603–621.
- Matsumura, Y.; Minowa, T.; Potic, B.; Kersten, S.R.A.; Prins, W.; Van Swaaij, W.P.M.; Van De Beld, B.; Elliott, D.C.; Neuenschwander, G.G.; Kruse, A.; et al. Biomass gasification in near- and super-critical water: Status and prospects. Biomass Bioenergy 2005, 29, 269–292.
- Kruse, A. Supercritical water gasification. Biofuels Bioprod. Biorefin. 2008, 2, 415–437.
- Fan, C.; Jin, H. A numerical study on gasification of a single char particle in supercritical water for hydrogen production. Fuel 2020, 268, 117399.
- Wen, Q.L.; Gu, H.Y. Numerical simulation of heat transfer deterioration phenomenon in supercritical water through vertical tube. Ann. Nucl. Energy 2010, 37, 1272–1280.
- Gutiérrez Ortiz, F.J.; Kruse, A. The use of process simulation in supercritical fluids applications. React. Chem. Eng. 2020, 5, 424–451.
- Pinkard, B.R.; Gorman, D.J.; Tiwari, K.; Kramlich, J.C.; Reinhall, P.G.; Novosselov, I.V. Review of Gasification of Organic Compounds in Continuous-Flow, Supercritical Water Reactors. Ind. Eng. Chem. Res. 2018, 57, 3471–3481.
- Rodriguez Correa, C.; Kruse, A. Supercritical water gasification of biomass for hydrogen production—Review. J. Supercrit. Fluids 2018, 133, 573–590.
- Ferreira-Pinto, L.; Silva Parizi, M.P.; de Araújo, P.C.C.S.; Zanette, A.F.; Cardozo-Filho, L. Experimental basic factors in the production of H2 via supercritical water gasification. Int. J. Hydrogen Energy 2019, 44, 25365–25383.
- Ibrahim, A.B.A.; Akilli, H. Supercritical water gasification of wastewater sludge for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 10328–10349.
- Zhang, Y.; Li, L.; Xu, P.; Liu, B.; Shuai, Y.; Li, B. Hydrogen production through biomass gasification in supercritical water: A review from exergy aspect. Int. J. Hydrogen Energy 2019, 44, 15727–15736.
- Hu, Y.; Gong, M.; Xing, X.; Wang, H.; Zeng, Y.; Xu, C.C. Supercritical water gasification of biomass model compounds: A review. Renew. Sustain. Energy Rev. 2020, 118, 12–14.
- Gutiérrez Ortiz, F.J. Techno-economic assessment of supercritical processes for biofuel production. J. Supercrit. Fluids 2020, 160, 104788.
- Abdpour, S.; Santos, R.M. Recent advances in heterogeneous catalysis for supercritical water oxidation/gasification processes: Insight into catalyst development. Process Saf. Environ. Prot. 2021, 149, 169–184.
- Farobie, O.; Matsumura, Y.; Syaftika, N.; Amrullah, A.; Hartulistiyoso, E.; Bayu, A.; Moheimani, N.R.; Karnjanakom, S.; Saefurahman, G. Recent advancement on hydrogen production from macroalgae via supercritical water gasification. Bioresour. Technol. Rep. 2021, 16, 100844.
- Lee, C.S.; Conradie, A.V.; Lester, E. Review of supercritical water gasification with lignocellulosic real biomass as the feedstocks: Process parameters, biomass composition, catalyst development, reactor design and its challenges. Chem. Eng. J. 2021, 415, 128837.
- Okolie, J.A.; Epelle, E.I.; Nanda, S.; Castello, D.; Dalai, A.K.; Kozinski, J.A. Hydrogen Production: A Comprehensive Review. J. Supercrit. Fluids 2021, 173, 105199.
- Matsumura, Y.; Minowa, T. Fundamental design of a continuous biomass gasification process using a supercritical water fluidized bed. Int. J. Hydrogen Energy 2004, 29, 701–707.
- Müller, J.B.; Vogel, F. Tar and coke formation during hydrothermal processing of glycerol and glucose. Influence of temperature, residence time and feed concentration. J. Supercrit. Fluids 2012, 70, 126–136.
- Lu, Y.; Zhang, T.; Dong, X. Bed to wall heat transfer in supercritical water fluidized bed: Comparison with the gas-solid fluidized bed. Appl. Therm. Eng. 2014, 88, 297–305.
- Zhang, T.; Lu, Y.; Yao, L. Experimental study of wall-to-bed heat transfer in a supercritical water fluidized bed. Int. J. Multiph. Flow 2018, 109, 26–34.
- Wu, Z.; Ren, Y.; Ou, G.; Jin, H. Influence of special water properties variation on the heat transfer of supercritical water flow around a sphere. Chem. Eng. Sci. 2020, 222, 115698.
- Liang, X.; Zhao, Q.; Dong, Y.; Guo, L.; Jin, Z.; Liu, Q. Experimental investigation on supercritical water gasification of organic-rich shale with low maturity for syngas production. Energy Fuels 2021, 35, 7657–7665.
- Sinag, A.; Kruse, A.; Maniam, P. Hydrothermal conversion of biomass and different model compounds. J. Supercrit. Fluids 2012, 71, 80–85.
- Chen, Y.; Guo, L.; Jin, H.; Yin, J.; Lu, Y.; Zhang, X. An experimental investigation of sewage sludge gasification in near and super-critical water using a batch reactor. Int. J. Hydrogen Energy 2013, 38, 12912–12920.
- Castello, D.; Rolli, B.; Kruse, A.; Fiori, L. Supercritical water gasification of biomass in a ceramic reactor: Long-time batch experiments. Energies 2017, 10, 1734.
- Graz, Y.; Bostyn, S.; Richard, T.; Bocanegra, P.E.; De Bilbao, E.; Poirier, J.; Gokalp, I. Hydrothermal conversion of Ulva macro algae in supercritical water. J. Supercrit. Fluids 2016, 107, 182–188.
- Weijin, G.; Zizheng, Z.; Yue, L.; Qingyu, W.; Lina, G. Hydrogen production and phosphorus recovery via supercritical water gasification of sewage sludge in a batch reactor. Waste Manag. 2019, 96, 198–205.
- Demirel, E.; Erkey, C.; Ayas, N. Supercritical water gasification of fruit pulp for hydrogen production: Effect of reaction parameters. J. Supercrit. Fluids 2021, 177, 105329.
- Weiss-Hortala, E.; Kruse, A.; Ceccarelli, C.; Barna, R. Influence of phenol on glucose degradation during supercritical water gasification. J. Supercrit. Fluids 2010, 53, 42–47.
- Goodwin, A.K.; Rorrer, G.L. Reaction rates for supercritical water gasification of xylose in a micro-tubular reactor. Chem. Eng. J. 2010, 163, 10–21.
- Zhang, L.; Champagne, P.; Xu, C.C. Supercritical water gasification of an aqueous by-product from biomass hydrothermal liquefaction with novel Ru modified Ni catalysts. Bioresour. Technol. 2011, 102, 8279–8287.
- Gutiérrez Ortiz, F.J.; Serrera, A.; Galera, S.; Ollero, P. Experimental study of the supercritical water reforming of glycerol without the addition of a catalyst. Energy 2013, 56, 193–206.
- Castello, D.; Kruse, A.; Fiori, L. Low temperature supercritical water gasification of biomass constituents: Glucose/phenol mixtures. Biomass Bioenergy 2015, 73, 84–94.
- Nanda, S.; Reddy, S.N.; Hunter, H.N.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of fructose as a model compound for waste fruits and vegetables. J. Supercrit. Fluids 2015, 104, 112–121.
- Ferreira-Pinto, L.; Feirhrmann, A.C.; Corazza, M.L.; Fernandes-Machado, N.R.C.; Dos Reis Coimbra, J.S.; Saldaña, M.D.A.; Cardozo-Filho, L. Hydrogen production and TOC reduction from gasification of lactose by supercritical water. Int. J. Hydrogen Energy 2015, 40, 12162–12168.
- Gutiérrez Ortiz, F.J.; Campanario, F.J.; Ollero, P. Supercritical water reforming of model compounds of bio-oil aqueous phase: Acetic acid, acetol, butanol and glucose. Chem. Eng. J. 2016, 298, 243–258.
- Adar, E.; Ince, M.; Bilgili, M.S. Supercritical water gasification of sewage sludge by continuous flow tubular reactor: A pilot scale study. Chem. Eng. J. 2020, 391, 123499.
- Bergmann, C.M.; Ormiston, S.J.; Chatoorgoon, V. Comparative study of turbulence model predictions of upward supercritical fluid flow in vertical rod bundle subchannels. Nucl. Eng. Des. 2017, 322, 177–191.
- Jaromin, M.; Anglart, H. A numerical study of heat transfer to supercritical water flowing upward in vertical tubes under normal and deteriorated conditions. Nucl. Eng. Des. 2013, 264, 61–70.
- Shang, Z.; Chen, S. Numerical investigation of diameter effect on heat transfer of supercritical water flows in horizontal round tubes. Appl. Therm. Eng. 2011, 31, 573–581.
- Huang, X.; Wang, Q.; Song, Z.; Yin, Y.; Wang, H. Heat transfer characteristics of supercritical water in horizontal double-pipe. Appl. Therm. Eng. 2020, 173, 115191.
- El-Morshedy, S.E.D.; Ibrahim, S.M.A.; Alyan, A.; Abdelmaksoud, A. Heat transfer deterioration mechanism for water at supercritical pressure. Int. J. Thermofluids 2020, 7–8, 100020.
- Gao, Z.; Bai, J. Numerical analysis on nonuniform heat transfer of supercritical pressure water in horizontal circular tube. Appl. Therm. Eng. 2017, 120, 10–18.
- Liu, L.; Xiao, Z.; Yan, X.; Zeng, X.; Huang, Y. Heat transfer deterioration to supercritical water in circular tube and annular channel. Nucl. Eng. Des. 2013, 255, 97–104.
- Dongliang, M.; Tao, Z.; Bing, L.; Ali Shahzad, M.; Yanping, H. An improved correlation on the onset of heat transfer deterioration in supercritical water. Nucl. Eng. Des. 2018, 326, 290–300.
- Xie, J.; Yan, H.; Sundén, B.; Xie, G. A numerical prediction on heat transfer characteristics from a circular tube in supercritical fluid crossflow. Appl. Therm. Eng. 2019, 153, 692–703.
- Wang, Z.; Qi, G.; Li, M. Numerical investigation of heat transfer to supercritical water in vertical tube under semicircular heating condition. Energies 2019, 12, 3958.
- Zhang, L.; Cai, B.; Weng, Y.; Gu, H.; Wang, H.; Li, H.; Chatoorgoon, V. Experimental investigations on flow characteristics of two parallel channels in a forced circulation loop with supercritical water. Appl. Therm. Eng. 2016, 106, 98–108.
- Basu, P.; Mettanant, V. Biomass Gasification in Supercritical Water—A Review. Int. J. Chem. React. Eng. 2009, 7.
- Odu, S.O.; Koster, P.; Van Der Ham, A.G.J.; Van Der Hoef, M.A.; Kersten, S.R.A. Heat Transfer to Sub- and Supercritical Water Flowing Upward in a Vertical Tube at Low Mass Fluxes: Numerical Analysis and Experimental Validation. Ind. Eng. Chem. Res. 2016, 55, 13120–13131.
- Kawasaki, S.I.; Sue, K.; Ookawara, R.; Wakashima, Y.; Suzuki, A.; Hakuta, Y.; Arai, K. Engineering study of continuous supercritical hydrothermal method using a T-shaped mixer: Experimental synthesis of NiO nanoparticles and CFD simulation. J. Supercrit. Fluids 2010, 54, 96–102.
- Qu, M.; Yang, D.; Liang, Z.; Wan, L.; Liu, D. Experimental and numerical investigation on heat transfer of ultra-supercritical water in vertical upward tube under uniform and non-uniform heating. Int. J. Heat Mass Transf. 2018, 127, 769–783.
- Miwa, Y.; Noishiki, K.; Suzuki, T.; Takatsuki, K. Research and Development Kobe Steel Engineering Reports; Kobe Steel Ltd.: Kobe, Japan, 2013; pp. 23–27.
- Mortean, M.V.V.; Cisterna, L.H.R.; Paiva, K.V.; Mantelli, M.B.H. Development of diffusion welded compact heat exchanger technology. Appl. Therm. Eng. 2016, 93, 995–1005.
- Sarmiento, A.P.C.; Soares, V.H.T.; Carqueja, G.G.; Batista, J.V.C.; Milanese, F.H.; Mantelli, M.B.H. Thermal performance of diffusion-bonded compact heat exchangers. Int. J. Therm. Sci. 2020, 153, 106384.
- Vacuum Process Engineering Diffusion Bonded Microchannel Heat Exchangers. Available online: https://www.vpei.com/diffusion-bonded-microchannel-heat-exchangers/ (accessed on 9 November 2021).
- Kobe Steel Ltd. Diffusion Bonded Compact Heat Exchanger. Available online: https://www.kobelco.co.jp/english/products/ecmachinery/dche/files/Microchannel_dche_smcr_brochure.pdf (accessed on 9 November 2021).
- Stankiewicz, A.; Moulijn, J. Re-Engineering the Chemical Processing Plant. Process Intensification; Marcel Dekker, Inc.: New York, NY, USA, 2004; ISBN 0824743024.