Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by zhijian Han.
In humans, Interleukin-8 (IL-8 or CXCL8) is a granulocytic chemokine with multiple roles within the tumor microenvironment (TME), such as recruiting immunosuppressive cells to the tumor, increasing tumor angiogenesis, and promoting epithelial-to-mesenchymal transition (EMT).
- CXCL8-CXCR1/2 axis
- cancer
- tumor microenviroment
- immunotherapy
- interleukin-8
1. CXCL8-CXCR1/2 Signaling Pathway
The proinflammatory cytokine CXCL8 was initially found as a chemotactic agent for neutrophils in inflammatory diseases. Through both autocrine and paracrine signaling, the CXCL8-CXCR1/2 axis can recruit the neutrophil to clear bacteria and protect the host from infection. Due to the similarity of the pathogenesis of inflammatory diseases and cancer, more researchers have focused on the roles of the CXCL8-CXCR1/2 axis in cancer [22][1].
CXCL8 is a peptide with 72 amino acids and has a critical N-terminal motif of Glu-Leu-Arg (ELR). CXCL8 can be secreted by fibroblasts, CAFs, endothelial cells, epithelial cells, DCs, monocytes, macrophages, and cancer cells [23][2]. CXCL8 signals through two cell-surface receptors of CXCR1 and CXCR2. CXCR1 and CXCR2 are the G-protein-coupled receptor for a group of C-X-C chemokines. CXCR1 interacts with CXCL6 and CXCL8, whereas CXCR2 interacts with CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8. CXCL8-CXCR1/2 signaling with CAF, the microbiome, and immune cell help in recruiting granulocytes such as neutrophils and MDSCs to the site of the TME and contribute to tumor growth by enhancing angiogenesis and promoting cancer cell proliferation [24][3] and immune resistance [25][4] (Figure 1).
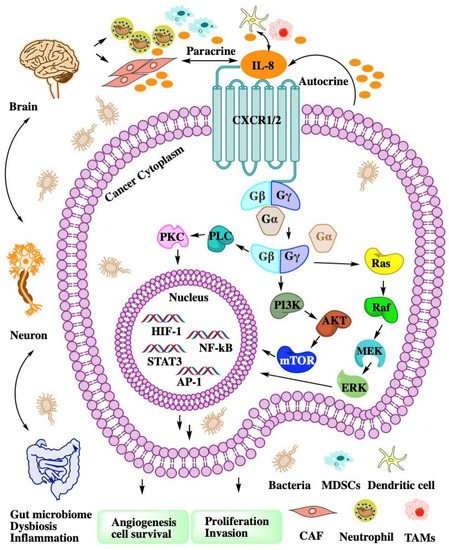
Figure 1. The intricate network of the CXCL8-CXCR1/2 axis in TME. CXCL8 binding to CXCR1/2 activates G-protein-mediated signaling cascades in cancer cell. CXCR1/2 activation leads to the dissociation of the Gα subunit from the Gβγ subunits. The signal of Gβγ subunits activate kinase to enhance angiogenesis, proliferation, and invasion. Cancer cell autocrine CXCL8 to recruit MDSCs or neutrophil to TME. Dysbiosis or inflammation affects myeloid cell recruitment through the gut–brain axis.
2. Interactions of the CXCL8-CXCR1/2 Axis and CAF
CAFs are activated fibroblast populations and the major cellular components of the TME in primary and metastatic cancers [26,27][5][6]. CAFs have different features from normal fibroblasts after infiltrating tumor tissue, such as enhanced proliferation and epigenetic changes to produce secreted factors [28][7]. CAFs are contributors to desmoplasia that can facilitate tumorigenicity, cancer cell proliferation, metastasis, and cancer immunotherapy resistance through complex interactions and intricate signaling [29][8] with other cell types in the TME [30][9].
The population of CAFs are highly heterogeneous because several progenitor cell types can be reprogrammed into CAFs [31][10]. Quiescent or resident fibroblasts are major progenitor cells of CAFs [32][11]. Hepatic [33][12] or pancreatic stellate cells [34][13] are the putative origin of CAFs. Adipocytes [35][14], endothelial cells [36][15], epithelial cells [37][16], and bone marrow cells [38][17] can be reprogrammed into CAFs. CAFs can also derive from multiple resident precursors, such as smooth muscle cells or mesenchymal stem cells [39][18]. Nevertheless, the precise origins of CAFs remain elusive because of the lack of lineage biomarkers.
CAFs have both tumor-promoting and tumor-suppressive functions [40][19]. The tumor-suppressive functions of CAFs [41][20] remain poorly understood. Part of the host defensive mechanism involves promotion of anticancer immunity, tumor-inhibitory signaling, tumor-restraining metabolism, and ECM-related physical barriers to tumor cell invasion and dissemination. Desmoplasia is the growth of fibrous or connective tissue in desmoplastic breast, lung, and pancreatic cancers. The desmoplastic reaction may form the desmoplasia or dense fibrosis around the tumor to restrict its growth and migration [42][21]. The mechanisms of tumor-promoting roles of CAFs are mainly regulatory functions via growth factors, cytokines, and chemokines contributing to angiogenesis; ECM remodeling; aberrant stroma, and an immunosuppressive TME [43][22]. Mass cytometry and single-cell analysis of pancreatic tumors and healthy pancreas samples found two stable and functionally distinct pancreatic fibroblast lineages. CD105-positive pancreatic fibroblasts promote tumor growth, but CD105-negative fibroblasts are highly tumor suppressive in a manner dependent on adaptive immunity [44][23].
CAF subsets have specialized secretory functions, such as cytokines, chemokines, and ECM molecules collagen I, which contribute to ECM remodeling and immunomodulatory function. Tumor–fibroblast interactions via soluble factors determine the final outcome of the tumorigenic process and affect the cancer therapy [45][24]. The CAF population is heterogeneous according to the cell origins, and the functional heterogeneity can be regulated by paracrine molecules such as CXCL8 and CXCR1/2 ligands [30][9]. CAFs can attract monocytes by secreting CXCL8 to enhance TAM enrichment and suppress NK cells’ function in colorectal cancer [46][25]. In gastric cancer tissues of chemoresistant patients, CXCL8 was highly expressed and located in CAFs by immunohistochemistry assay. A high serum CXCL8 level was associated with poor response to cisplatin therapy in gastric cancer patients [47][26]. CAFs are major sources of chemokines (CXCL1 and CXCL8) that recruit granulocytes (TAM and PMN-MDSC) to tumors. Combining a selective CSF1R inhibitor (JNJ-40346527) with a CXCR2 antagonist (SB225002) blocked granulocyte recruitment and demonstrated a strong antitumor effect, which was further improved by the addition of antiprogrammed cell death protein 1 (PD-1) [48][27].
Chronic inflammation and proinflammatory cytokines tumor necrosis factor α (TNFα) and interleukin 1β (IL-1β) can induce the conversion of MSCs to inflammatory CAFs. These CAFs secrete prometastatic chemokines including CXCL6 and CXCL8 in Luminal-A breast cancer cells and enhance migration [49][28]. CXCL8 can induce normal ovarian fibroblasts to CAFs and stimulate xenograft tumor growth in mice. The ovarian cancer cell stemness was promoted by the CXCL8 secreted from CAFs through the Notch3 signaling pathway [50][29]. Gastric cancer extracellular vesicles transfer various miRNAs and induce chemokines such as CXCL1 and CXCL8 expression in CAFs. Aberrant chemokine CXCL1 and CXCL8 expression in CAFs was closely associated with tumor progression and poorer survival in gastric cancer patients [51][30]. CAFs demonstrate a high level of basal secretory autophagy in head and neck squamous cell carcinoma. Secretory autophagy is involved in the export of cellular inflammatory mediators such as IL-6 and CXCL8. Combination therapy using autophagy inhibition with cisplatin significantly reduced tumor volume [52][31].
Stromal cells such as CAFs and MSCs enhance the triple-negative subtype of breast cancer metastasis-related phenotypes, including angiogenesis, migratory, and invasive properties, by releasing inflammatory chemokines such as CCL2 and CXCL8 [53][32]. Notch 1 activation is required for the induction of CXCL8 in tumor–stroma interactions and consequently for prometastatic activities [54][33]. Androgen receptor signaling in CAFs affects prostate cancer cell migration mediated by CXCL8 and CCL2 [55][34]. CAFs express CXCR2 and respond to paracrine signals of pancreatic cancer cells by upregulating CXCR2 ligands such as CXCL1, CXCL7, and CXCL8. CXCR2 knockout in a pancreatic ductal adenocarcinoma syngeneic mouse model suppressed angiogenesis and induced an antitumor response, but increased CAF activation, fibrosis, and metastasis in a mutation-dependent manner [56][35].
The CAF response to chemotherapy is highly variable and may impact on cancer therapy outcome [57][36]. Traditional maximum-tolerated dose chemotherapy induces CAF activation and results in the expression and secretion of ELR motif-positive chemokines such as CXCL6 and CXCL8 [58][37]. These chemokines signal though CXCR2 on cancer cells to transform into tumor-initiating cells, thus promoting aggression and treatment resistance. While the same overall dose administered as a low-dose metronomic chemotherapy largely prevented CAF activation and enhanced treatment response, it improved survival in mice [58][37]. CAF-secreted IL-6 and CXCL8 induce Bromodomain-containing protein 4 (BRD4) protein expression and lead to chromatin remodeling and Bromodomain and Extraterminal (BET) inhibitor resistance in colorectal cancer (CRC). Inhibition of IL-6/CXCL8-JAK2 signaling sensitized BET inhibitors in a CRC mouse xenograft model [59][38]. Simultaneous blocking of IL-6 and CXCL8 can inhibit CAF-induced human melanoma cell invasiveness using neutralizing antibodies in a 3D spheroid invasion assay [60][39]. Senescent human fibroblasts can secrete IL-6 and CXCL8 to promote cancer cell invasion and metastasis [61][40]. Senescent CAFs are a pathologically relevant fibroblast population that secrete excess CXCL8 to promote pancreatic cancer invasion [62][41]. A new subset of CD10+GPR77+CAFs induce cancer stem cells’ (CSCs) enrichment and chemoresistance by secreting IL-6 and CXCL8 in cancer. CD10+GPR77+CAFs constitute a supporting niche for CSCs, and its high expression correlates with poor survival in breast and lung cancer patients [63][42].
3. Therapeutic Targeting of the CXCL8-CXCR1/2 Axis in Cancer
Chemokine CXCL8 activates both CXCR1 and CXCR2. All of them have been shown to be upregulated in acute or chronic inflammatory diseases including cancer. CXCR1 and CXCR2 constitute the primary mechanism for the recruitment of neutrophils and MDSCs, which could enhance tumor progression and suppress immune therapy efficacy. Blocking the CXCL8-CXCR1/2 axis with small molecules (Figure 2) or antibodies should be a promising therapeutic strategy to overcome immune suppression in the TME.
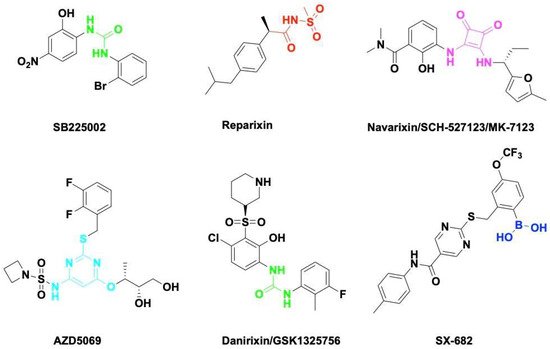
Figure 2. Small molecule antagonists targeting CXCR1/2. Blocking CXCR1 or CXCR2 impairs immune suppressive cell recruitment and angiogenesis to enhance cancer therapy efficacy.
SB225002 was first reported as a small molecule CXCL8 inhibitor binding to CXCR2. SB225002 selectively blocked CXCL8-induced neutrophil chemotaxis and margination in rabbits [164][43]. Blocking CXCR2 with SB225002 has proved to inhibit tumor progression in breast cancer [165][44], ovarian cancer [166][45], acute myeloid leukemia [167][46], and nasopharyngeal carcinoma [168][47]. Reparixin is a non-competitive allosteric inhibitor of CXCR1 and CXCR2 that could inhibit polymorphonuclear cell recruitment in vivo. The activity of Reparixin on CXCR1 was 100-fold higher than on CXCR2 [169][48]. CXCR1 has been identified as a druggable target for breast cancer stem cells [170][49].
Navarixin is an orally selective, CXCR1 and CXCR2 receptor antagonist that impairs neutrophil recruitment in rodents and monkeys [171][50]. Navarixin decreased tumor cell proliferation and angiogenesis in a melanoma mouse model [172][51]. A phase II clinical trial (NCT03473925) assessed the efficacy and safty of navarixin in combination with anti-PD1 pembrolizumab in NSCLC, Castration-Resistant Prostate Cancer and Colorectal Cancer (Table 1). AZD5069 is a reversible CXCR2 antagonist that inhibits CXCL8 binding and neutrophil chemotaxis [173][52]. AZD5069 treatment inhibits TAM infiltration and vessel formation, increases CD4+/CD8+ T-cell infiltration, and suppresses tumor growth in advanced prostate cancer [174][53]. Combination therapy of AZD5069 with anti-PD1 Durvalumab (MEDI4736) was studied in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (NCT02499328) and metastatic pancreatic ductal adenocarcinoma (NCT02583477). Danirixin is also a reversible and selective antagonist of CXCR2 that may be of benefit in diseases of excess neutrophilia [175][54]. The clinical trial of danirixin in healthy, elderly human volunteers demonstrated that it was safe and tolerable, and no serious adverse events were reported [176,177][55][56]. A recent study showed that danirixin suppressed breast cancer migration, invasion, and metastasis mediated by CXCL8 and TAMs [178][57].
Table 1. Selected clinical trials targeted in CXCL8-CXCR1/2 axis.
| Drug (Manufacturer) | Target | Therapeutic Combinations | Cancer Type | Phase | Clinical Trials |
Recruitment Status |
|---|---|---|---|---|---|---|
| SX-682 (Syntrix Biosystems, Inc.) | CXCR1/2 | Pembrolizumab (anti-PD-1) | Metastatic Melanoma | Phase I | NCT03161431 | Recruiting |
| Nivolumab (anti-PD-1) | Metastatic Colorectal Carcinoma | Phase Ib/II | NCT04599140 | Recruiting | ||
| Nivolumab (anti-PD-1) | Pancreatic Cancer | Phase I | NCT04477343 | Recruiting | ||
| Bintrafusp alfa (anti-PD-L1/TGF-β) CV301 (cancer vaccine) |
Advanced Solid Tumors | Phase I | NCT04574583 | Active, not recruiting | ||
| AZD5069 (AstraZeneca) | CXCR2 | Durvalumab (anti-PD-L1) | Advanced Solid Tumors | Phase Ib/II | NCT02499328 | Active, not recruiting |
| Durvalumab (anti-PD-L1) | Metastatic Pancreatic Ductal Adenocarcinoma | Phase II | NCT02583477 | Completed | ||
| Navarixin (Merck Sharp & Dohme Corp.) | CXCR1/2 | Pembrolizumab (anti-PD-1) | Advanced Solid Tumors | Phase II | NCT03473925 | Completed |
| HuMax-IL8 (Bristol-Myers Squiibb) | CXCL8 | Nivolumab (anti-PD-1) | Head and Neck Squamous Cell Carcinoma | Phase II | NCT04848116 | Recruiting |
| Nivolumab (anti-PD-1) | Prostate Cancer | Phase Ib/II | NCT03689699 | Recruiting | ||
| Nivolumab (anti-PD-1) | Pancreatic Cancer | Phase II | NCT02451982 | Recruiting | ||
| Nivolumab (anti-PD-1) | Hepatocellular Carcinoma | Phase II | NCT04050462 | Recruiting | ||
| Nivolumab (anti-PD-1) | Advanced Cancers | Phase I/II | NCT03400332 | Recruiting | ||
| Nivolumab (anti-PD-1) | Non-small Cell Lung Cancer | Phase II | NCT04123379 | Recruiting |
SX-682 is a novel CXCR1/2 chemokine receptor boronic acid antagonist with potential anticancer activities. Orally bioavailable SX-682 enhanced NK cell activation and therapeutic efficacy by inhibiting MDSC accumulation in the TME [93][58]. A combination of SX-682 with anti-PD1 caused a reduction in tumor burden b7 increasing CD8+ T-cell infiltration and decreasing neutrophil accumulation in non-small cell lung cancer [179][59]. Treatment of tumor-bearing mice with SX682 reduced intertumoral MDSCs, increased CD8+ T-cell recruitment, and inhibited tumor growth in melanoma and breast cancer [180][60]. Several clinical trials were designed to assess the efficacy of SX-682 in combination with anti-PD1 Nivolumab or Pembrolizumab in metastatic melanoma, colorectal carcinoma, and pancreatic cancer (Table 1). Fully humanized neutralizing antibody ABX-IL8 inhibits angiogenesis, tumor growth, and metastasis in melanoma [181][61] and bladder cancer [182][62] through downregulation of MMP-2. HuMax-IL8(BMS-986253) is another fully human anti-CXCL8 monoclonal antibody. A phase I clinical trial of HuMax-IL8 (NCT02536469) showed no objective tumor responses, but it is safe and well tolerated [183][63]. Phase II clinical trials of HuMax-IL8 plus anti-PD1 Nivolumab are ongoing in patients with advanced solid tumors (Table 1).
Chimeric antigen receptor (CAR) T-cell therapy has shown clinical efficacy for hematological malignancies but still remains a challenge for solid tumors [184][64]. The major obstacles of CAR-T therapy in solid tumors are tumor heterogeneity, an immunosuppressive TME, and T-cell trafficking/infiltrating to the tumor. CXCR1/2-modified CAR-T cells enhance T-cell trafficking, persistence of T cells in the tumor, long-lasting immunologic memory, and therapy efficacy in aggressive solid tumors such as glioblastoma, ovarian, and pancreatic cancer [185][65].
4. CXCL8 as a Prognosis Biomarker in Cancer Therapy
Tumor burden (or tumor size) predicts response to immunotherapy in patients with cancer. A large TME and a small TME are characterized by different cell populations and responses to specific interventions [186][66]. Radiologic imaging such as computed tomographhy (CT) is the most commonly used technology for tumor burden monitoring even though it has limitations for the evaluation of the response to immunotherapy. Functional imaging techniques [187][67] such as positron emission tomography (PET) or electron paramagnetic resonance (EPR) can sense the TME hypoxia and visualize tissue redox status non-invasively, which assists image-guided diagnoses and efficacy evaluations of cancer therapy [188][68]. CXCL8 serum concentrations can accurately reflect the tumor burden of patients following antitumor therapy and have prognostic significance [189][69].
CXCL8 could be a serum biomarker by which to predict clinical benefit from immune checkpoint blockade in melanoma and NSCLC patients. Serum CXCL8 levels significantly decreased in responding patients treated with anti-PD1 nivolumab or pembrolizumab, which were associated with longer overall survival [190][70]. In gemcitabine-refractory patients with pancreatic cancer, plasma CXCL8 is a useful circulating biomarker for predicting resistance to nanoliposomal irinotecan therapy [191][71]. Baseline IL-6/CXCL8 can predict objective response and overall survival in patients with advanced HCC treated with sorafenib [192][72].
References
- Alfaro, C.; Sanmamed, M.F.; Rodríguez-Ruiz, M.E.; Teijeira, Á.; Oñate, C.; González, Á.; Ponz, M.; Schalper, K.A.; Pérez-Gracia, J.L.; Melero, I. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat. Rev. 2017, 60, 24–31.
- Gonzalez-Aparicio, M.; Alfaro, C. Significance of the IL-8 pathway for immunotherapy. Hum. Vaccin. Immunother. 2020, 16, 2312–2317.
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol. Ther. 2021, 219, 107692.
- David, J.M.; Dominguez, C.; Hamilton, D.H.; Palena, C. The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines 2016, 4, 22.
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115.
- Öhlund, D.; Elyada, E.; Tuveson, D. Fibroblast heterogeneity in the cancer wound. J. Exp. Med. 2014, 211, 1503–1523.
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 218.
- Yoshida, G.J. Regulation of heterogeneous cancer-associated fibroblasts: The molecular pathology of activated signaling pathways. J. Exp. Clin. Cancer Res. 2020, 39, 112.
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804.
- Barrett, R.L.; Puré, E. Cancer-associated fibroblasts and their influence on tumor immunity and immunotherapy. Elife 2020, 9, e57243.
- Arina, A.; Idel, C.; Hyjek, E.M.; Alegre, M.L.; Wang, Y.; Bindokas, V.P.; Weichselbaum, R.R.; Schreiber, H. Tumor-associated fibroblasts predominantly come from local and not circulating precursors. Proc. Natl. Acad. Sci. USA 2016, 113, 7551–7556.
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411.
- Thomas, D.; Radhakrishnan, P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol. Cancer 2019, 18, 14.
- Bochet, L.; Lehuédé, C.; Dauvillier, S.; Wang, Y.Y.; Dirat, B.; Laurent, V.; Dray, C.; Guiet, R.; Maridonneau-Parini, I.; Le Gonidec, S.; et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013, 73, 5657–5668.
- Zeisberg, E.M.; Potenta, S.; Xie, L.; Zeisberg, M.; Kalluri, R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007, 67, 10123–10128.
- Willis, B.C.; duBois, R.M.; Borok, Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc. Am. Thorac Soc. 2006, 3, 377–382.
- Quante, M.; Tu, S.P.; Tomita, H.; Gonda, T.; Wang, S.S.; Takashi, S.; Baik, G.H.; Shibata, W.; Diprete, B.; Betz, K.S.; et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 2011, 19, 257–272.
- Spaeth, E.L.; Dembinski, J.L.; Sasser, A.K.; Watson, K.; Klopp, A.; Hall, B.; Andreeff, M.; Marini, F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE 2009, 4, e4992.
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598.
- Helms, E.; Onate, M.K.; Sherman, M.H. Fibroblast Heterogeneity in the Pancreatic Tumor Microenvironment. Cancer Discov. 2020, 10, 648–656.
- Zeltz, C.; Primac, I.; Erusappan, P.; Alam, J.; Noel, A.; Gullberg, D. Cancer-associated fibroblasts in desmoplastic tumors: Emerging role of integrins. Semin. Cancer Biol. 2020, 62, 166–181.
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186.
- Hutton, C.; Heider, F.; Blanco-Gomez, A.; Banyard, A.; Kononov, A.; Zhang, X.; Karim, S.; Paulus-Hock, V.; Watt, D.; Steele, N.; et al. Single-cell analysis defines a pancreatic fibroblast lineage that supports antitumor immunity. Cancer Cell 2021, 39, 1227–1244.e20.
- Mishra, P.; Banerjee, D.; Ben-Baruch, A. Chemokines at the crossroads of tumor-fibroblast interactions that promote malignancy. J. Leukoc. Biol. 2011, 89, 31–39.
- Zhang, R.; Qi, F.; Zhao, F.; Li, G.; Shao, S.; Zhang, X.; Yuan, L.; Feng, Y. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis. 2019, 10, 273.
- Zhai, J.; Shen, J.; Xie, G.; Wu, J.; He, M.; Gao, L.; Zhang, Y.; Yao, X.; Shen, L. Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019, 454, 37–43.
- Kumar, V.; Donthireddy, L.; Marvel, D.; Condamine, T.; Wang, F.; Lavilla-Alonso, S.; Hashimoto, A.; Vonteddu, P.; Behera, R.; Goins, M.A.; et al. Cancer-Associated Fibroblasts Neutralize the Antitumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors. Cancer Cell 2017, 32, 654–668.e5.
- Rubinstein-Achiasaf, L.; Morein, D.; Ben-Yaakov, H.; Liubomirski, Y.; Meshel, T.; Elbaz, E.; Dorot, O.; Pichinuk, E.; Gershovits, M.; Weil, M.; et al. Persistent Inflammatory Stimulation Drives the Conversion of MSCs to Inflammatory CAFs That Promote Pro-Metastatic Characteristics in Breast Cancer Cells. Cancers 2021, 13, 1472.
- Ji, Z.; Tian, W.; Gao, W.; Zang, R.; Wang, H.; Yang, G. Cancer-Associated Fibroblast-Derived Interleukin-8 Promotes Ovarian Cancer Cell Stemness and Malignancy Through the Notch3-Mediated Signaling. Front. Cell Dev. Biol. 2021, 9, 684505.
- Naito, Y.; Yamamoto, Y.; Sakamoto, N.; Shimomura, I.; Kogure, A.; Kumazaki, M.; Yokoi, A.; Yashiro, M.; Kiyono, T.; Yanagihara, K.; et al. Cancer extracellular vesicles contribute to stromal heterogeneity by inducing chemokines in cancer-associated fibroblasts. Oncogene 2019, 38, 5566–5579.
- New, J.; Arnold, L.; Ananth, M.; Alvi, S.; Thornton, M.; Werner, L.; Tawfik, O.; Dai, H.; Shnayder, Y.; Kakarala, K.; et al. Secretory Autophagy in Cancer-Associated Fibroblasts Promotes Head and Neck Cancer Progression and Offers a Novel Therapeutic Target. Cancer Res. 2017, 77, 6679–6691.
- Katanov, C.; Lerrer, S.; Liubomirski, Y.; Leider-Trejo, L.; Meshel, T.; Bar, J.; Feniger-Barish, R.; Kamer, I.; Soria-Artzi, G.; Kahani, H.; et al. Regulation of the inflammatory profile of stromal cells in human breast cancer: Prominent roles for TNF-α and the NF-κB pathway. Stem Cell Res. Ther. 2015, 6, 87.
- Liubomirski, Y.; Lerrer, S.; Meshel, T.; Morein, D.; Rubinstein-Achiasaf, L.; Sprinzak, D.; Wiemann, S.; Körner, C.; Ehrlich, M.; Ben-Baruch, A. Notch-Mediated Tumor-Stroma-Inflammation Networks Promote Invasive Properties and CXCL8 Expression in Triple-Negative Breast Cancer. Front. Immunol. 2019, 10, 804.
- Cioni, B.; Nevedomskaya, E.; Melis, M.H.M.; van Burgsteden, J.; Stelloo, S.; Hodel, E.; Spinozzi, D.; de Jong, J.; van der Poel, H.; de Boer, J.P.; et al. Loss of androgen receptor signaling in prostate cancer-associated fibroblasts (CAFs) promotes CCL2- and CXCL8-mediated cancer cell migration. Mol. Oncol. 2018, 12, 1308–1323.
- Awaji, M.; Saxena, S.; Wu, L.; Prajapati, D.R.; Purohit, A.; Varney, M.L.; Kumar, S.; Rachagani, S.; Ly, Q.P.; Jain, M.; et al. CXCR2 signaling promotes secretory cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. FASEB J. 2020, 34, 9405–9418.
- Sonnenberg, M.; van der Kuip, H.; Haubeis, S.; Fritz, P.; Schroth, W.; Friedel, G.; Simon, W.; Mürdter, T.E.; Aulitzky, W.E. Highly variable response to cytotoxic chemotherapy in carcinoma-associated fibroblasts (CAFs) from lung and breast. BMC Cancer 2008, 8, 364.
- Chan, T.S.; Hsu, C.C.; Pai, V.C.; Liao, W.Y.; Huang, S.S.; Tan, K.T.; Yen, C.J.; Hsu, S.C.; Chen, W.Y.; Shan, Y.S.; et al. Metronomic chemotherapy prevents therapy-induced stromal activation and induction of tumor-initiating cells. J. Exp. Med. 2016, 213, 2967–2988.
- Wang, W.; Tang, Y.A.; Xiao, Q.; Lee, W.C.; Cheng, B.; Niu, Z.; Oguz, G.; Feng, M.; Lee, P.L.; Li, B.; et al. Stromal induction of BRD4 phosphorylation Results in Chromatin Remodeling and BET inhibitor Resistance in Colorectal Cancer. Nat. Commun. 2021, 12, 4441.
- Jobe, N.P.; Rösel, D.; Dvořánková, B.; Kodet, O.; Lacina, L.; Mateu, R.; Smetana, K.; Brábek, J. Simultaneous blocking of IL-6 and IL-8 is sufficient to fully inhibit CAF-induced human melanoma cell invasiveness. Histochem. Cell Biol. 2016, 146, 205–217.
- Orjalo, A.V.; Bhaumik, D.; Gengler, B.K.; Scott, G.K.; Campisi, J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. USA 2009, 106, 17031–17036.
- Wang, T.; Notta, F.; Navab, R.; Joseph, J.; Ibrahimov, E.; Xu, J.; Zhu, C.Q.; Borgida, A.; Gallinger, S.; Tsao, M.S. Senescent Carcinoma-Associated Fibroblasts Upregulate IL8 to Enhance Prometastatic Phenotypes. Mol. Cancer Res. 2017, 15, 3–14.
- Su, S.; Chen, J.; Yao, H.; Liu, J.; Yu, S.; Lao, L.; Wang, M.; Luo, M.; Xing, Y.; Chen, F.; et al. CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 2018, 172, 841–856.e16.
- White, J.R.; Lee, J.M.; Young, P.R.; Hertzberg, R.P.; Jurewicz, A.J.; Chaikin, M.A.; Widdowson, K.; Foley, J.J.; Martin, L.D.; Griswold, D.E.; et al. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J. Biol. Chem. 1998, 273, 10095–10098.
- Kim, S.; You, D.; Jeong, Y.; Yoon, S.Y.; Kim, S.A.; Kim, S.W.; Nam, S.J.; Lee, J.E. WNT5A augments cell invasiveness by inducing CXCL8 in HER2-positive breast cancer cells. Cytokine 2020, 135, 155213.
- Yung, M.M.; Tang, H.W.; Cai, P.C.; Leung, T.H.; Ngu, S.F.; Chan, K.K.; Xu, D.; Yang, H.; Ngan, H.Y.; Chan, D.W. GRO-α and IL-8 enhance ovarian cancer metastatic potential via the CXCR2-mediated TAK1/NFκB signaling cascade. Theranostics 2018, 8, 1270–1285.
- Cheng, J.; Li, Y.; Liu, S.; Jiang, Y.; Ma, J.; Wan, L.; Li, Q.; Pang, T. CXCL8 derived from mesenchymal stromal cells supports survival and proliferation of acute myeloid leukemia cells through the PI3K/AKT pathway. FASEB J. 2019, 33, 4755–4764.
- Liu, X.; Lan, T.; Mo, F.; Yang, J.; Wei, Y.; Wei, X. Antitumor and Radiosensitization Effects of a CXCR2 Inhibitor in Nasopharyngeal Carcinoma. Front. Cell Dev. Biol. 2021, 9, 689613.
- Bertini, R.; Allegretti, M.; Bizzarri, C.; Moriconi, A.; Locati, M.; Zampella, G.; Cervellera, M.N.; Di Cioccio, V.; Cesta, M.C.; Galliera, E.; et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: Prevention of reperfusion injury. Proc. Natl. Acad. Sci. USA 2004, 101, 11791–11796.
- Ruffini, P.A. The CXCL8-CXCR1/2 Axis as a Therapeutic Target in Breast Cancer Stem-Like Cells. Front. Oncol. 2019, 9, 40.
- Chapman, R.W.; Minnicozzi, M.; Celly, C.S.; Phillips, J.E.; Kung, T.T.; Hipkin, R.W.; Fan, X.; Rindgen, D.; Deno, G.; Bond, R.; et al. A novel, orally active CXCR1/2 receptor antagonist, Sch527123, inhibits neutrophil recruitment, mucus production, and goblet cell hyperplasia in animal models of pulmonary inflammation. J. Pharm. Exp. Ther. 2007, 322, 486–493.
- Singh, S.; Sadanandam, A.; Nannuru, K.C.; Varney, M.L.; Mayer-Ezell, R.; Bond, R.; Singh, R.K. Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, and angiogenesis. Clin. Cancer Res. 2009, 15, 2380–2386.
- Nicholls, D.J.; Wiley, K.; Dainty, I.; MacIntosh, F.; Phillips, C.; Gaw, A.; Mårdh, C.K. Pharmacological characterization of AZD5069, a slowly reversible CXC chemokine receptor 2 antagonist. J. Pharm. Exp. Ther. 2015, 353, 340–350.
- Di Mitri, D.; Mirenda, M.; Vasilevska, J.; Calcinotto, A.; Delaleu, N.; Revandkar, A.; Gil, V.; Boysen, G.; Losa, M.; Mosole, S.; et al. Re-education of Tumor-Associated Macrophages by CXCR2 Blockade Drives Senescence and Tumor Inhibition in Advanced Prostate Cancer. Cell Rep. 2019, 28, 2156–2168.e5.
- Busch-Petersen, J.; Carpenter, D.C.; Burman, M.; Foley, J.; Hunsberger, G.E.; Kilian, D.J.; Salmon, M.; Mayer, R.J.; Yonchuk, J.G.; Tal-Singer, R. Danirixin: A Reversible and Selective Antagonist of the CXC Chemokine Receptor 2. J. Pharm. Exp. Ther. 2017, 362, 338–346.
- Miller, B.E.; Smart, K.; Mistry, S.; Ambery, C.L.; Bloomer, J.C.; Connolly, P.; Sanderson, D.; Shreeves, T.; Smith, R.; Lazaar, A.L. The pharmacokinetics of conventional and bioenhanced tablet formulations of danirixin (GSK1325756) following oral administration in healthy, elderly, human volunteers. Eur. J. Drug Metab. Pharm. 2014, 39, 173–181.
- Miller, B.E.; Mistry, S.; Smart, K.; Connolly, P.; Carpenter, D.C.; Cooray, H.; Bloomer, J.C.; Tal-Singer, R.; Lazaar, A.L. The pharmacokinetics and pharmacodynamics of danirixin (GSK1325756)—A selective CXCR2 antagonist–in healthy adult subjects. BMC Pharm. Toxicol. 2015, 16, 18.
- Nie, G.; Cao, X.; Mao, Y.; Lv, Z.; Lv, M.; Wang, Y.; Wang, H.; Liu, C. Tumor-associated macrophages-mediated CXCL8 infiltration enhances breast cancer metastasis: Suppression by Danirixin. Int. Immunopharmacol. 2021, 95, 107153.
- Greene, S.; Robbins, Y.; Mydlarz, W.K.; Huynh, A.P.; Schmitt, N.C.; Friedman, J.; Horn, L.A.; Palena, C.; Schlom, J.; Maeda, D.Y.; et al. Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clin. Cancer Res. 2020, 26, 1420–1431.
- Kargl, J.; Zhu, X.; Zhang, H.; Yang, G.H.Y.; Friesen, T.J.; Shipley, M.; Maeda, D.Y.; Zebala, J.A.; McKay-Fleisch, J.; Meredith, G.; et al. Neutrophil content predicts lymphocyte depletion and anti-PD1 treatment failure in NSCLC. JCI Insight 2019, 4, e130850.
- Yang, J.; Yan, C.; Vilgelm, A.E.; Chen, S.C.; Ayers, G.D.; Johnson, C.A.; Richmond, A. Targeted Deletion of CXCR2 in Myeloid Cells Alters the Tumor Immune Environment to Improve Antitumor Immunity. Cancer Immunol. Res. 2021, 9, 200–213.
- Huang, S.; Mills, L.; Mian, B.; Tellez, C.; McCarty, M.; Yang, X.D.; Gudas, J.M.; Bar-Eli, M. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am. J. Pathol. 2002, 161, 125–134.
- Mian, B.M.; Dinney, C.P.; Bermejo, C.E.; Sweeney, P.; Tellez, C.; Yang, X.D.; Gudas, J.M.; McConkey, D.J.; Bar-Eli, M. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin. Cancer Res. 2003, 9, 3167–3175.
- Bilusic, M.; Heery, C.R.; Collins, J.M.; Donahue, R.N.; Palena, C.; Madan, R.A.; Karzai, F.; Marté, J.L.; Strauss, J.; Gatti-Mays, M.E.; et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J. Immunother. Cancer 2019, 7, 240.
- Hou, A.J.; Chen, L.C.; Chen, Y.Y. Navigating CAR-T cells through the solid-tumour microenvironment. Nat. Rev. Drug Discov. 2021, 20, 531–550.
- Jin, L.; Tao, H.; Karachi, A.; Long, Y.; Hou, A.Y.; Na, M.; Dyson, K.A.; Grippin, A.J.; Deleyrolle, L.P.; Zhang, W.; et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat. Commun. 2019, 10, 4016.
- Kim, S.I.; Cassella, C.R.; Byrne, K.T. Tumor Burden and Immunotherapy: Impact on Immune Infiltration and Therapeutic Outcomes. Front. Immunol. 2020, 11, 629722.
- Kayser, A.S. Functional imaging. Handb. Clin. Neurol. 2019, 163, 61–72.
- Matsumoto, K.I.; Mitchell, J.B.; Krishna, M.C. Multimodal Functional Imaging for Cancer/Tumor Microenvironments Based on MRI, EPRI, and PET. Molecules 2021, 26, 1614.
- Sanmamed, M.F.; Carranza-Rua, O.; Alfaro, C.; Oñate, C.; Martín-Algarra, S.; Perez, G.; Landazuri, S.F.; Gonzalez, A.; Gross, S.; Rodriguez, I.; et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin. Cancer Res. 2014, 20, 5697–5707.
- Sanmamed, M.F.; Perez-Gracia, J.L.; Schalper, K.A.; Fusco, J.P.; Gonzalez, A.; Rodriguez-Ruiz, M.E.; Oñate, C.; Perez, G.; Alfaro, C.; Martín-Algarra, S.; et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann. Oncol. 2017, 28, 1988–1995.
- Merz, V.; Zecchetto, C.; Santoro, R.; Simionato, F.; Sabbadini, F.; Mangiameli, D.; Piro, G.; Cavaliere, A.; Deiana, M.; Valenti, M.T.; et al. Plasma IL8 Is a Biomarker for TAK1 Activation and Predicts Resistance to Nanoliposomal Irinotecan in Patients with Gemcitabine-Refractory Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 4661–4669.
- Öcal, O.; Schütte, K.; Kupčinskas, J.; Morkunas, E.; Jurkeviciute, G.; de Toni, E.N.; Ben Khaled, N.; Berg, T.; Malfertheiner, P.; Klümpen, H.J.; et al. Baseline Interleukin-6 and -8 predict response and survival in patients with advanced hepatocellular carcinoma treated with sorafenib monotherapy: An exploratory post hoc analysis of the SORAMIC trial. J. Cancer Res. Clin. Oncol. 2021.
More
