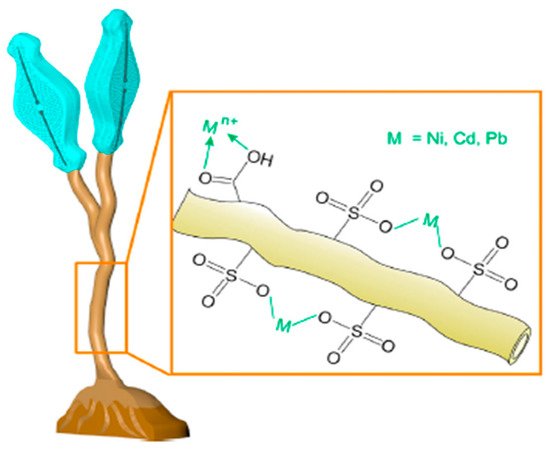
Didymosphenia geminata is a species of freshwater diatom that is known as invasive and is propagating quickly around the world. The polysaccharide-based stalks of D. geminata enable versatile potential applications and use as a biopolymer, in drug delivery and wound healing, and as biocompatible scaffolding in cell adhesion and proliferation. Furthermore, the polysaccharide nature of stalks and their metal-adsorption capacity allow them to have excellent wastewater remediation potential.
- chitin
- diatoms
- didymo
- invasive species
- polysaccharide structure
- drug delivery
- wound dressing
1. Introduction
2. Applications of D. geminata as a Biomaterial
D. geminata is a relatively new finding and has many potential uses in biomedicine and as a biomaterial for drug delivery, cell adhesion, and proliferation [6]. he polysaccharides in the stalk of D. geminata appear to be primarily sulfated xylogalactan, and the stalk was found to be intrinsically hydrophilic, a trait that is one of the first determiners of the extent of protein adsorption [14]. The presence of sulfate groups within polysaccharides shows an improvement in the immune system and would be beneficial to the nutraceutical industry [15]. The intrinsic biocompatible nature of D. geminata is attributed to its similarity in structure to glycosaminoglycans, which are a vital component in the tissue extracellular matrix [16].
Zglobicka in 2013 examined how previous studies had cast stalks of D. geminata as capable of adhering to multiple kinds of substrates because they were built in concentric circles of materials of variable compositions [17]. The chemical composition of the stalk seems to largely be unknown, but it is agreed that the main composition is amorphous silica [17][18]. The use of silica and biosilica in bone regeneration is well studied. It is understood to improve bone regeneration and increase bone density and is thought to play a role in the stabilization of collagen in the bone matrix [19]. The specific structure of D. geminata and its diatom frustules made of silica are especially beneficial with regard to drug delivery due to characteristics such as high permeability and low density [20]. D. geminata also exhibits anticancer properties [2]. As seen in Figure 12, the diatom has a nanosized porous silica capsule, which allows controlled drug release. The drug itself can be added to the external and internal surface of the diatom and then released based on need. The drug can be loaded onto the nanoparticles directly through noncovalent interactions like electrostatic bonding, hydrogen bonding, van der Waals forces, pi–pi stacking, and hydrophobic interactions [21].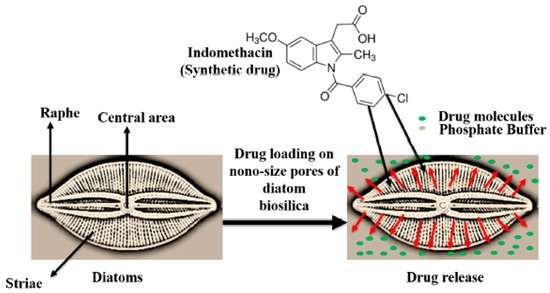
3. D. geminata Applications in Wastewater Treatment
4. D. geminata’s Economic Impacts
References
- Whitton, B.A.; Ellwood, N.T.W.; Kawecka, B. Biology of the Freshwater Diatom Didymosphenia: A Review. Hydrobiologia 2009, 630, 1–37.
- Hussein, H.A.; Abdullah, M.A. Anticancer Compounds Derived from Marine Diatoms. Mar. Drugs 2020, 18, 356.
- Sundareshwar, P.V.; Upadhayay, S.; Abessa, M.; Honomichl, S.; Berdanier, B.; Spaulding, S.A.; Sandvik, C.; Trennepohl, A. Didymosphenia geminata: Algal Blooms in Oligotrophic Streams and Rivers. Geophys. Res. Lett. 2011, 38.
- Wysokowski, M.; Bartczak, P.; Żółtowska-Aksamitowska, S.; Chudzińska, A.; Piasecki, A.; Langer, E.; Bazhenov, V.V.; Petrenko, I.; Noga, T.; Stelling, A.L.; et al. Adhesive Stalks of Diatom Didymosphenia geminata as a Novel Biological Adsorbent for Hazardous Metals Removal. CLEAN-Soil Air Water 2017, 45, 1600678.
- Ladrera, R.; Gomà, J.; Prat, N. Effects of Didymosphenia geminata Massive Growth on Stream Communities: Smaller Organisms and Simplified Food Web Structure. PLoS ONE 2018, 13, e0193545.
- Ejaz, H.; Somanader, E.; Dave, U.; Ehrlich, H.; Rahman, M.A. Didymo and Its Polysaccharide Stalks: Beneficial to the Environment or Not? Polysaccharides 2021, 2, 69–79.
- Elwell, L.C.; Gillis, C.-A.; Kunza, L.A.; Modley, M.D. Management Challenges of Didymosphenia geminata. Diatom Res. 2014, 29, 303–305.
- Taylor, B.W.; Bothwell, M.L. The Origin of Invasive Microorganisms Matters for Science, Policy, and Management: The Case of Didymosphenia geminata. Bioscience 2014, 64, 531–538.
- Brand, C.; Grech, M. Recent Invasion of Didymosphenia geminata (Lyngbye) M. Schmidt in a Patagonian Regulated River Promotes Changes in Composition and Density of Macroinvertebrate Community. Biol. Invasions 2020, 22, 1903–1915.
- Jellyman, P.G.; Harding, J.S. Disentangling the Stream Community Impacts of Didymosphenia geminata: How Are Higher Trophic Levels Affected? Biol. Invasions 2016, 18, 3419–3435.
- Jones, L.R.; Manrique, J.M.; Uyua, N.M.; Whitton, B.A. Genetic Analysis of the Invasive Alga Didymosphenia geminata in Southern Argentina: Evidence of a Pleistocene Origin of Local Lineages. Sci. Rep. 2019, 9, 18706.
- Bothwell, M.L.; Taylor, B.W.; Kilroy, C. The Didymo Story: The Role of Low Dissolved Phosphorus in the Formation of Didymosphenia geminata Blooms. Diatom Res. 2014, 29, 229–236.
- Kilroy, C.; Bothwell, M. Environmental Control of Stalk Length in the Bloom-Forming, Freshwater Benthic Diatom Didymosphenia geminata (Bacillariophyceae). J. Phycol. 2011, 47, 981–989.
- Bothwell, M.L.; Spaulding, S.A. Synopsis of the 2007 International Workshop on Didymosphenia geminate. In Proceedings of the 2007 International Workshop on Didymosphenia geminate; Fisheries and Oceans Canada: Ottawa, ON, Canada, 2008; 96p.
- Figueroa, F.A.; Abdala-Díaz, R.; Hernández, V.; Pedreros, P.; Aranda, M.; Cabrera-Pardo, J.R.; Pérez, C.; Becerra, J.; Urrutia, R. Invasive Diatom Didymosphenia geminata as a Source of Polysaccharides with Antioxidant and Immunomodulatory Effects on Macrophage Cell Lines. J. Appl. Phycol. 2020, 32, 93–102.
- Wahab, I.F.; Razak, S.I. Polysaccharides as Composite Biomaterials. In Composites from Renewable and Sustainable Materials; InteahOpen: London, UK, 2016; pp. 65–84.
- Zgłobicka, I. Aspects of Structural Biology of Didymosphenia geminata (Lyngb.) M. Schmidt (Bacillariophyta). IJA 2013, 15, 291–310.
- Zglobicka, I. Frustules of Didymosphenia geminata as a Modifier of Resins. Mater. Eng. 2018, 1, 10–16.
- Arora, M.; Arora, E. The Promise of Silicon: Bone Regeneration and Increased Bone Density. J. Arthrosc. Jt. Surg. 2017, 4, 103–105.
- Zgłobicka, I. Exploratory Study of the Use of Didymosphenia geminata Stalks as a Functional Biomaterial. Ph.D. Thesis, Division of Materials Design, The Warsaw University of Technology, Warszawa, Poland, 2015.
- Wang, N.; Cheng, X.; Li, N.; Wang, H.; Chen, H. Nanocarriers and their loading strategies. Adv. Healthc. Mater. 2019, 8, 1801002.
- Andryukov, B.G.; Besednova, N.N.; Kuznetsova, T.A.; Zaporozhets, T.S.; Ermakova, S.P.; Zvyagintseva, T.N.; Chingizova, E.A.; Gazha, A.K.; Smolina, T.P. Sulfated Polysaccharides from Marine Algae as a Basis of Modern Biotechnologies for Creating Wound Dressings: Current Achievements and Future Prospects. Biomedicines 2020, 8, 301.
- Ehrlich, H.; Motylenko, M.; Sundareshwar, P.V.; Ereskovsky, A.; Zgłobicka, I.; Noga, T.; Płociński, T.; Tsurkan, M.V.; Wyroba, E.; Suski, S.; et al. Multiphase Biomineralization: Enigmatic Invasive Siliceous Diatoms Produce Crystalline Calcite. Adv. Funct. Mater. 2016, 26, 2503–2510.
- Na, Y.; Lee, J.; Lee, S.H.; Kumar, P.; Kim, J.H.; Patel, R. Removal of Heavy Metals by Polysaccharide: A Review. Polym.-Plast. Technol. Mater. 2020, 59, 1770–1790.
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential Use of Algae for Heavy Metal Bioremediation, a Critical Review. J. Environ. Manag. 2016, 181, 817–831.
- Reinoso-Guerra, E.; Aristizabal, J.; Arce, B.; Zurob, E.; Dennett, G.; Fuentes, R.; Suescún, A.V.; Cárdenas, L.; da Cunha, T.H.R.; Cabezas, R.; et al. Nanostructured Didymosphenia geminata-Based Membrane for Efficient Lead Adsorption from Aqueous Solution. J. Environ. Chem. Eng. 2021, 9, 105269.
- Ubando, A.T.; Africa, A.D.M.; Maniquiz-Redillas, M.C.; Culaba, A.B.; Chen, W.-H.; Chang, J.-S. Microalgal Biosorption of Heavy Metals: A Comprehensive Bibliometric Review. J. Hazard. Mater. 2021, 402, 123431.
- Arbabi, M.; Hemati, S.; Amiri, M. Removal of Lead Ions from Industrial Wastewater: A Review of Removal Methods. Int. J. Epidemiol. Res. 2015, 2, 105–109.
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Vardhan, K.H.; SundarRajan, P. Microalgae for Biofuel Production and Removal of Heavy Metals: A Review. Environ. Chem. Lett. 2020, 18, 1905–1923.
- Zgłobicka, I.; Chlanda, A.; Woźniak, M.; Łojkowski, M.; Szoszkiewicz, R.; Mazurkiewicz-Pawlicka, M.; Święszkowski, W.; Wyroba, E.; Kurzydłowski, K.J. Microstructure and Nanomechanical Properties of Single Stalks from Diatom Didymosphenia geminata and Their Change Due to Adsorption of Selected Metal Ions. J. Phycol. 2017, 53, 880–888.
- Rosaen, A.L.; Grover, E.A.; Spencer, C.W. The Costs of Aquatic Invasive Species to Great Lakes States; Anderson Economic Group: East Lansing, MI, USA, 2016; 51p.
- Beville, S.T.; Kerr, G.N.; Hughey, K.F.D. Valuing Impacts of the Invasive Alga Didymosphenia geminata on Recreational Angling. Ecol. Econ. 2012, 82, 1–10.
- Bergey, E.A.; Cooper, J.T.; Phillips, B.C. Substrate Characteristics Affect Colonization by the Bloom-Forming Diatom Didymosphenia geminata. Aquat. Ecol. 2010, 44, 33–40.
- Ministère du Développement Durable, de L’environnement et des Parcs; Ministère des Ressources Naturelles et de la Faune. What Is Didymo and How Can We Prevent It from Spreading in Our Rivers? Ministère du Développement Durable, de l’Environnement et des Parcs: Rouyn-Noranda, QC, Canada, 2008; 13p.
- Government of Canada, Fisheries and Oceans Canada. A Canadian Action Plan to Address the Threat of Aquatic Invasive Species. Available online: https://www.dfo-mpo.gc.ca/species-especes/publications/ais-eae/plan/page01-eng.html (accessed on 24 October 2021).
