Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Chunpeng Wan and Version 2 by Catherine Yang.
Citrus fruits are subjected to a diversity of postharvest diseases caused by various pathogens during picking, packing, storage and transportation. Green and blue molds, caused by Penicillium digitatum and Penicillium italicum, respectively, are two major postharvest citrus diseases and cause significant economic losses during the commercialization phase.
- plant-derived fungicides
- Penicillium digitatum
- Penicillium italicum
1. Medicinal Plant Extracts
Different medicinal plants have been reported to possess unique uses in the prevention and treatment of diseases in China. Over 5000 different medicinal plants have been reported in Chinese medical system, which mostly bear strong antifungal properties. Herbs and edible plants have a long history of being used as Citrus preservatives due to their low toxicity, safety and easy acceptance by consumers. In recent years, many scholars have selected a large number of extracts from herbal medicines that inhibit the activity of P. digitatum and P. italicum. The effect of rosemary (Rosmarinus officinalis L.) extracts on the growth of the green mold of Citrus was studied by Hendel et al. [1][9], and in vitro antifungal assays showed a clear inhibitory effect of the rosemary essential oils and methanol extract on the growth of P. digitatum. An aqueous crude extract prepared from lyophilized leaves of Solanum nigrum showed an inhibitory effect on P. digitatum [2][10] with a remarkable inhibition zone. Solanum nigrum extracts showed an important preventive antifungal effect where 100% of inhibition was observed at 7 days of storage.
The antifungal activity of medicinal plant extracts is affected by many factors such as origin, season of harvest, processing method and extraction conditions, etc. In order to make extracts that exert optimal antifungal activity, some studies reported the effects of variable extraction conditions on the antifungal activity of plant extracts, and optimized the extraction procedures of some plants with antifungal effects.
2. Edible Plants Extracts
Many of the edible plants also have good antifungal effects and can potentially inhibit the growth of postharvest pathogenic fungi in Citrus fruits. The extracts of edible plants contain some edible flavorings. Aqueous and 20% ethanolic extracts of garlic (Family Amaryllidaceae) plant showed a significant inhibition of P. digitatum and P. italicum [3][17]. The inhibitory effect of 9 edible wild herbs were studied on common mold of fruits and vegetables, and we found that both Sanguisorba minor and Plantago lanceolata could significantly inhibit the germination of P. digitatum and P. italicum spores and the extension of germ tubes [4][18]. This occurrence is possibly related to the presence of caffeic acid derivatives and flavonoids in extracts.
Aloe vera (Family Liliaceae) is rich in polysaccharides, proteins, vitamins, active enzymes, lignin, anthraquinones and many other compounds. The anthraquinones are a characteristic component of aloe plant. Moreover, aloe extracts have a wide range of activities to inhibit microbial growth, while aloe saponins and anthraquinones were found as the active ingredients. The extracts were prepared from the aloe gel [5][6][23,24], which significantly inhibited the mycelial growth and spore germination of P. italicum. The antifungal activity of 8 different aloe species were also examined [7][25], out of them A. ferox, A. mitriformis and A. saponaria showed the highest inhibitory effects on P. digitatum and P. italicum, primarily due to the presence of a major component aloin in aloe gel.
Tea saponins are natural non-ionic surfactants isolated from Camellia sinensis and are widely used in foods, medicines, pesticides and its emulsifiers. Tea saponins can potentially improve the antifungal activity of Citrus chemical fungicides and biocontrol bacteria as well as affecting Citrus preservation. A strain of Bacillus amyloliquefaciens isolated and identified as HF-01 was found antagonistic to P. italicum from the epidermis of seedless granulated oranges [8][67]. in vitro experiments shown that the inhibitory rate of the antagonistic bacteria against the P. digitatum and P. italicum were above 96%, the use of HF-01 antagonistic bacteria alone has a certain antiseptic and fresh-keeping effect on the seedless sugar orange, but there is a significant difference compared with the chemical fungicide imazalil. At the same time, 50 μg/mL of tea saponins can significantly improve the fresh-keeping effect of HF-01 antagonistic bacteria (stronger than the activity of the two agents alone), which is not significantly different from chemical fungicides, and has no effect on fruit quality. This indicated that tea saponin and HF-01 have synergistic effects on antagonistic antifungal action. In addition, it was also found that tea saponins have a synergistic effect with other chemical fungicides such as imazalil and prochloraz [9][26].
3. Citrus Extracts
Citrus itself contains many antifungal substances (Figure 1, Table 2). The presence of essential oils extracted from Citrus peel, leaves and flowers by water distillation has strong antifungal effects on both the fungal strains P. digitatum and P. italicum [10][51]. Further studies have shown that the Citrus peel contains a large number of flavonoids, coumarins and volatile oils, which have the activity to resist the postharvest pathogenic fungi of Citrus fruits. Citrus peel itself also has a certain ability to resist pathogenic fungi, mainly through endogenous and induced (phytoalexins) antimicrobial substances. Citrus coumarins are a class of phytoalexins that inhibit the growth of postharvest pathogenic fungi. Phytoalexins refer to the kind of a substance that inhibits the growth of pathogenic fungi when the pathogenic fungi has invaded the fruits. It means this is the substance that is related to plant resistance to disease. After the Citrus fruit is treated by physical (exposure to γ-ray, ultraviolet, heat treatment, skin damage etc.) or chemical (pathogenic fungi, fungicides, etc.) agents, the skin of the Citrus fruit can induce some plant antitoxin (mainly coumarins). These phytoalexins are low or absent in normal Citrus. When Citrus is treated by physical or chemical agents, these phytoalexins can be produced or their presence was significantly increased. These phytoalexins significantly inhibit the growth of pathogenic microorganisms.
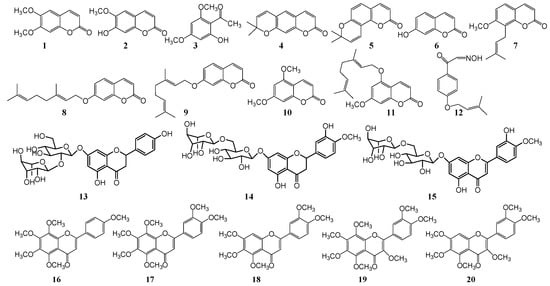
Figure 1.
Chemical structures of antifungal constituents 1–20.
Table 2.
Inhibitory activity of
Citrus
extracts on
P. digitatum
and
P. italicum.
| Citrus | Plants | Other name | Pathogens | Antifungal Constituents | References |
|---|
| C. aurantium | sour orange | P. citrophthora | , | P. italicum | and | Geotrichum | essential oils, polymethoxyflavones, tangeritin, nobiletin, sinensetin, heptamethoxyflavone and quercetogetin | [10][11] | [51,52] | |||||||
| C. paradisi | L. | grapefruit | P. digitatum | and | P. italicum | coumarins including Scoparone, seselin, umbelliferone, osthol, auraptene 7-geranoxycoumarin; essential oils, limonene, α-pinene, sabinene, myrcene, α-terpineol, linalool, citral, nootkatone | [12][13][14]] | [53,54 | [ | ,55 | 15] | ,56 | [16] | ,57 | [17 | ,58] |
| C. japonica | kumquat | P. digitatum | scoparone and scopoletin | [18] | [59] | |||||||||||
| C. sinensis | sweet orange | P. citrophthora | , | P. italicum | and | Geotrichum | scoparone and scopoletin, volatile oil, limonene, α-pinene, sabinene, myrcene, α-terpineol, linalool, citral; polymethoxyflavones | [11][17][19][20] | [52,58,60,61] | |||||||
| C. limon | lemon | P. citrophthora | , | P. digitatum | and | P. italicum | waxy components, hexane extract, scoparone, xanthoxylin and xanthyletin; limettin, isopimpinellin, 5-geranoxy-7-methoxycoumarin and Scoparone; volatile oil, citral | [20][21][22][23][24] | [61 | [ | ,62 | 25 | ,63,64 | ] | ,65,66] | |
| C. reticulata | mandarin | P. digitatum | waxy components, hexane extract, tangeritin, nobiletin | [20] | [61] | |||||||||||
| C. clementina | clementine | P. citrophthora | , | P. italicum | and | Geotrichum | volatile oil, nobiletin, and sinensetin, heptamethoxyflavone, limonene, α-pinene, sabinene, myrcene, α-terpineol, linalool | [11][17] | [52,58] |
Scoparone (1) is the most intensively studied induced plant phytoalexin to date. While it is present in smaller quantities in normal conditions, following exposure to inoculation of pathogens, heat treatment, ultraviolet and γ-rays irradiation etc., its contents significantly increase. The degree of increase is closely related to the conditions of treatment (temperature, energy and time of irradiation), the variety of Citrus and the degree of maturity. In general, the content of lemon, kumquat and ponkan is higher, and the content of immature fruit is lower than ripe fruits [26][27][28][29][68,69,70,71]. Scoparone (1) was first isolated from the grapefruit [12][53] peel by γ-rays treated. This compound was subsequently isolated from the rind and bark [30][31][32][72,73,74] of other Citrus inoculated with Phytophthora citrophthora. Its inhibitory activity against P. digitatum and P. italicum was studied with EC50 of 64 μg/mL and 60 μg/mL respectively. Scoparone (1) and scopoletin (2) were also isolated from the peels of kumquat and C. sinensis [18][19][59,60], and their content was significantly increased by short-wave ultraviolet irradiation C (UV-C). The peak was reached after 4-10 days of irradiation, and then decreased rapidly, which showed that irradiation can improve the storage stability of kumquat and C. sinensis.
In addition to scoparone (1), some other antifungal components of coumarin were isolated and identified from Citrus. Two phytoalexins—xanthoxylin (3) and xanthyletin (4) were isolated from the lemon bark [21][62] and roots [22][63] inoculated with P. citrophthora. 60 μg/mL of xanthyletin (4) can completely inhibit the growth of P. citrophthora in Citrus. Meanwhile some phenolic components (3,4-dimethoxybenzaldehyde) with xanthyletin (4) can synergistically improve its antifungal activity. Seselin (5) was isolated from the Citrus roots [13][33][54,75] inoculated with P. citrophthora, which is a pyranocoumarin phytoalexin. However, this compound was not detected in other parts, indicating that seselin (5) mainly exists in the roots of Citrus trees. Several endogenous coumarins, umbelliferone (6), osthol (7), auraptene (8) and 7-geranoxycoumarin (9), were isolated from the peel of grapefruit [14][15][16][55,56,57], and result showed they have antifungal effects. Angioni et al. [16][57] further studied the synthetic method of 7-geranoxycoumarin (9), and obtained 7-geranoxycoumarin (9) through the synthesis of geranyl bromide and umbelliferone (6). The inhibitory activities of umbelliferone (6) and 7-geranoxycoumarin (9) against P. digitatum and P. italicum were compared. The EC50 values were 95 μg/mL and 110 μg/mL, 57 μg/mL and 43 μg/mL, indicating that the structure of umbelliferone (6) can be modified to improve the antifungal activity. Ben-Yehoshua et al. [23][24][25][64,65,66] also found that green-skinned lemons have lower decay rate than yellow-skinned lemons, and their antifungal active substances were studied. One induced and four endogenous antifungal substances in lemon peels were identified by TCL-bioautography, GC-MS and NMR. There are citral, scoparone (1), limettin (10), 5-geranoxy-7-methoxycoumarin (11) and isopimpinellin (32). The EC50 of citral, limettin (10) and 5-geranoxy-7-methoxycoumarin (11) inhibiting the elongation of P. digitatum sprouts was 170-242 μg/mL, 860-886 μg/mL and 1578 μg/mL, respectively. The content of limettin (10), which is the active ingredient of green-skinned lemons, is higher than that of yellow-skinned lemons. Therefore, the green-skinned lemon is more resistant to storage. Dubery et al. [34][76] identified a new inducible antifungal substance, 4-(3-methyl-2-butenoxy) isonitrosoacetophenone (12), from γ-ray treated Citrus sinensis and lemon peel.
In addition to coumarin, there are some flavonoids in Citrus peels. In particular, polymethoxy- flavones are also resistant to the attack of P. digitatum and P. italicum. The waxy components of various Citrus peel and hexane extract [20][61] have resistance effects on a variety of fungi and bacteria, and the MIC concentration of P. digitatum is inhibited below 1000 μg/mL. And separating and identifying three activities ingredient: tangeritin (16), nobiletin (17) and scoparone (1). Further studies [35][36][77,78] have shown that naringin (13) and hesperidin (14) of dihydrogen flavonoids, diosmin (15) of flavonoids, tangeritin (16), sinensetin (18), heptamethoxyflavone (19) and nobiletin (17) of polymethyl-flavonoids are closely related to the susceptibility of Citrus to P. digitatum. The high content of flavonoids in Citrus peels is resistant to the infection of Penicillium spp. Ultraviolet radiation (UV) not only affects the content of coumarin in Citrus peel, but also affects the flavonoids. Arcas et al. [37][79] studied the effect of ultraviolet radiation (UV) on the content of flavonoids in Citrus aurantium and the activity of P. digitatum. After five days of inoculation with P. digitatum, the orange was irradiated by ultraviolet irradiation, and the content of naringin (13) in the peel decreased, while the content of tangeritin (16) increased. The inhibitory activity of tangeritin (16) is stronger than that of naringin (13), which showed that ultraviolet irradiation treatment of C. aurantium can improve its antifungal activity.
4. Volatile Oil
Volatile oil, also known as essential oil, is a volatile oily liquid widely found in plants. It is widely found in Labiatae, Umbelliferae, Lauraceae, Myrtaceae, Rutaceae and Asteraceae families. The peels of the Citrus fruit also contain a large amount of volatile oil components.
In addition to flavonoids and coumarins mentioned above, Citrus peels also contain volatile oils (Figure 1). The results indicated that Citrus essential oils had a good effect on inhibiting the pathogenic fungi of Citrus postharvest diseases, which may be related to its natural resistance to pathogens during fruit growth and evolution. The main components of Citrus essential oils, including limonene (content over 50% [38][80]), myrcene, citral, α-pinene, β-pinene, α-terpineol, β-linalool, β-phellandrene and β-ocimene were analyzed by GC-MS. The α-terpineol, β-linalool inhibit the growth of P. digitatum and P. italicum. However, limonene, myrcene, α-pinene and β-pinene stimulate their growth [17][58], which may be one of the reasons why damaged Citrus are vulnerable to pathogenic fungus. C. aurantium and C. sinensis volatile oil [11][52] can inhibit the growth of Penicillium spp., isolated and identified several polymethoxyflavones: tangeritin (16), nobiletin (17), and sinensetin (18), heptamethoxyflavone (19) and quercetogetin (20), Citrus polymethoxyflavones can resist a variety of Citrus postharvest pathogens (including P. citrophthora, P. italicum and Geotrichum). Caccioni et al. [39][81] studied the relationship between the volatile oil components of various Citrus peels and the inhibition of the activity of P. digitatum and P. italicum. The antifungal activity of the volatile oils of citrange, lemon, grapefruit and tangerine was strong, while the activity of the volatile oil of sweet orange and sour orange was slightly weak. It was found that the antifungal activity of volatile oil from Citrus peel was related to the content of sesquiterpenes and total monoterpenes (except limonene). Sweet orange, sour orange and grapefruit contain more than 90% limonene, and the content of limonene in citrange, lemon and tangerine is about 70%.
In addition to volatile oils of Citrus plants, the volatile oils of other plants (Labiatae, Asteraceae and Lauraceae) are also effective at inhibiting Citrus postharvest pathogens fungi (Figure 2). The most active ones are volatile oils such as laurel, clove, oregano, cinnamon and mint. CO2 supercritical fluid extraction of laurel oil [40][27] has a good inhibitory effect on postharvest pathogenic fungi, and good results at a concentration of 200 μg/mL. The chemical components of laurel oil were analyzed by GC-MS, and mainly contains 1.8-cineole, linalool, terpineol acetate, methyl eugenol, linalyl acetate, eugenol (21), sabinene, β-pinene and α-terpineol. Yigit et al. [41][14] studied the inhibitory effect of volatile oils from five plant spices on P. digitatum and the preservation effect of Citrus. Cumin volatile oil can completely inhibit the growth of mycelium and spore germination. Coriander, rosemary, wild thyme and dill volatile oils also have a certain antifungal effect (mycelial growth inhibition rate is 70–80%). During in vivo experiments, 900 ppm thyme essential oil was found to have a 50% inhibition rate, and other volatile oils had no effect.
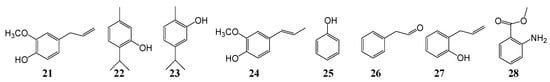
Figure 2.
Chemical structures of volatile antifungal constituents 21–28.
5. Other Plant Extracts
Some studies have also been conducted by scholars in screening other plant extracts that inhibit P. digitatum and P. italicum. Boubaker et al. [42][43][19,20] observed the activity of 71 kinds of Moroccan plant aqueous extracts inhibiting the P. digitatum and P. italicum, and found that most plants had a certain inhibitory effect, among which 15 plants had an inhibition rate of mycelium growth above 75% under experimental conditions. They were: Arenaria rubra, Anvillea radiata, Asteriscus graveolens, Bubonium odorum, Cistus villosus, Halimium umbellatum, Hammada scoparia, Ighermia pinifolia, Inula viscosa, Rubus ulmifolius, Thymus leptobotrys, Peganum harmala, Eucalyptus globulus, Sanguisorba minor and Ceratonia siliqua. At the same time, 10 mg/mL of A. graveolens, B. odorum and H. umbellatum extracts completely inhibited spore germination, and 1.2 mg/mL of T. leptobotrys volatile oil extract completely inhibited the growth of hyphae. They continued to study the antifungal activity of different solvent extracts from eight of the more active plants and found that A. radiata, A. graveolens, B. odorum, I. viscosa and T. leptobotrys were more active in petroleum ether, chloroform and ethyl acetate extracts. They also found that in the methanol extract of H. umbellatum, the petroleum ether extract of I. pinifolia and the chloroform extract of H. scoparia are highly active. Also, the polarities of the antifungal active substances of different plants are different [44][36]. Mexican desert plant extracts [45][38] also inhibit the action of postharvest fungi in fruits. The ethanol extracts of Lippia graveolens and Yucca filifera Chaub in 1000 μl/L had more than 90% inhibition effect on spore germination of P. digitatum, and the growth inhibition rate of mycelium was about 80%. Seven Jordanian plants [46][47][84,85] extracts also inhibit the action of P. digitatum and P. italicum. These were Fenugreek seeds, garlic, cinnamon, Peganum harmala, Inula viscosa and Solanum nigrum. Among them, garlic, cinnamon and Solanum nigrum methanolic extracts were the most active. Their IC50 is 3.75–18, 5.0–23 and 8.75–24.75 μg respectively. Qasem et al. [48][49][15,39] studied the inhibitory effects of some common weed aqueous extracts on three fungi. It was found that six plant extracts including Crepis aspera, Viscum cruciatum, Chenopodium murale, Sisymbrium irio, Solanum nigrum and Ranunculus asiaticus have a good inhibitory effect on P. digitatum in Citrus. Among them, the R. asiaticus can completely inhibit the growth of P. digitatum which has been cultured for up to 16 days. H. Boubaker et al. [50][37] studied the aerial parts of four Thymus species in Morocco and found that the main constituents of the extracts of these four plants were carvacrol (76.94%) for Thymus leptobotrys, borneol (27.71%) and thymol (18.47%) for Thymus satureioides subsp. pseudomastichina, camphor (46.17%) and α-terpineol (7.69%) for Thymus broussonnetii subsp. hannonis and carvacrol (32.24%), γ-terpinene (19.60%) and p-cymene (13.52%) for Thymus riatarum. The results indicated that T. leptobotrys essential oil displayed the highest bioactivity, completely inhibiting the spore germination of G. citri-aurantii at 250 μL/L and of P. digitatum and P. italicum at 500 μL/L. T. riatarum essential oil was able to completely inhibit the spore germination of G. citri-qurantii (2000 μL/L) and both Penicillium species used as target organisms (1000 μL/L). Sayago et al. [51][40] studied the inhibitory effect of 9 Argentine plant aqueous extracts on two common Citrus pathogenic fungi. Three plant extracts of Chuquiraga atacamensis, Parastrephia phyliciformis and Parastrephia lepidophylla have inhibitory effect on P. digitatum. The activity is related to the total phenolic content, of which P. lepidophylla is the most active, and its MIC and MFC are 400 mg/L and 600 mg/L, respectively. P. lepidophylla extract with a total polyphenol content of 600 mg/L and a mixture of fruit wax can reduce the rate of decay in the storage of lemons that have been damaged and inoculated with P. digitatum.
6. Targeted Isolation and Identification of Antifungal Active Ingredients
The antifungal effect of plant extracts is mainly due to the action of some components or the synergy of several components. It is important to determine the structure of the antifungal components in the plant extracts, while the structure-activity relationship (SAR) and quality control standards of extracts can be further studied. Some scholars have used column chromatography, HPLC-MS and GC-MS to identify active ingredients (Figure 3) in plant extracts with antifungal activity. Li et al. [52][41] isolated and identified the antifungal active constituents against Citrus pathogenic fungi from Radix Angelicae biseratae, and identified four coumarin components from the active fraction by GC-MS, namely isobergapten (29) and pimpinellin (30), sphondin (31) and isopimpinellin (32), which are the main antifungal active constituents.
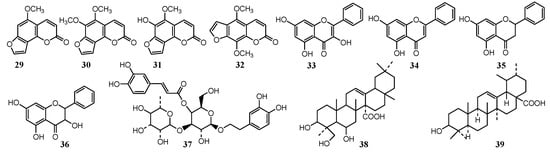
Figure 3.
Chemical structures of antifungal constituents 29–39.
Propolis extract has good antifungal, antiviral, antioxidation, scavenging free radical and anti-tumor activities. Propolis [53][86] ethyl acetate and ethanol extract can inhibit the spore germination of P. digitatum and P. italicum, and the mycelium is deformed to inhibit its growth, while the activity is related to the total flavonoid content. Propolis ethanol extract was extracted with systemic solvent, and the antifungal activity showed that ethyl acetate was the most active part. Four flavonoids [54][87], galangin (33), chrysin (34), pinocembrine (35) and pinobanksin (36), were identified by TCL- bio-autography, column chromatography separation and HPLC-MS technology. The galangin (33) was also identified from the Helichrysum aureonitens acetone extract [55][42] by antifungal activity tracking separation. P. digitatum and P. italicum were most sensitive to the 0.01 mg/mL concentration of galangin (33), and the inhibition rate is about 30%. Emmanuel et al. [56][43] studied the activity of two species of Lippia, Lippia javanica and Lippia rehmannii, suppressing a Guazatine-resistant strain of P. digitatum. The plant extract of 0.6 mg/mL could inhibit the growth of mycelium, and verbascoside (37) was identified as the main active ingredient.
Gao Kun et al. [57][45] studied the antifungal active ingredients of Astilbe myriantha Diels, and finally isolated and identified an active triterpenoid: 3β, 6β, 24-trihydroxyurs-12-en-27-oic acid (38), whose IC50 was 13.9 mg/L. Breonadia salicina acetone extract [58][46] has an inhibitory effect on variety of Penicillium, and the MIC concentration of inhibiting P. digitatum is 0.16 mg/mL, from which a major active triterpenoid composition i.e., ursolic acid (39) was isolated and identified.
