Fermentation continues to be the most common biotechnological tool to be used in
cereal-based beverages, as it is relatively simple and economical. Fermented beverages hold a long
tradition and have become known for their sensory and health-promoting attributes. Considering
the attractive sensory traits and due to increased consumer awareness of the importance of healthy
nutrition, the market for functional, natural, and non-alcoholic beverages is steadily increasing all over
the world. This paper outlines the current achievements and technological development employed to
enhance the qualitative and nutritional status of non-alcoholic fermented cereal beverages (NFCBs).
Following an in-depth review of various scientific publications, current production methods are
discussed as having the potential to enhance the functional properties of NFCBs and their safety, as a
promising approach to help consumers in their efforts to improve their nutrition and health status.
Moreover, key aspects concerning production techniques, fermentation methods, and the nutritional
value of NFCBs are highlighted, together with their potential health benefits and current consumption
trends. Further research efforts are required in the segment of traditional fermented cereal beverages
to identify new potentially probiotic microorganisms and starter cultures, novel ingredients as
fermentation substrates, and to finally elucidate the contributions of microorganisms and enzymes in
the fermentation process.
- cereal beverage
- fermentation
- functional
- non-alcoholic
- health benefits
1. Definition
Reviewing the existent literature regarding some of the most studied (Non-Alcoholic Fermented Cereal Beverage) NFCBs, it is shown that they are usually based on barley, maize, millet, oats, rice, rye, sorghum, and wheat [27,28][1][2]. Cereals are known as important sources of dietary proteins [29][3], energy, carbohydrates, vitamins, minerals, and fibre (arabinoxylan and β–glucan), but, at the same time, they are deficient in some basic components (essential amino acids, e.g., lysine)[4][5] [19,30]. Whole grains are known to have important bioactive compounds and high nutritional values and their regular consumption has a positive effect on health [31–33][6][7][8]. The presence of soluble fibre lowers the glycaemic index of the beverage by slowing down its digestion and absorption, whereas phenolic compounds have antioxidant potential and scavenge harmful free radicals in the body [9][9].
21. Introduction
Most of the traditional and currently produced NFCBs are considered functional foods and wholesome nutritional products [22,34,35][10][11][12]. Functional foods have been intensively studied in recent years and are continuously looked at as research efforts turn to processing food matrices, such as cereals, vegetables, and fruits, into medicine-like products.
A more detailed definition describes functional foods as industrially processed or natural foods that, when regularly consumed within a diverse diet at efficacious levels, have potentially positive effects on health outside basic nutrition [36][13]. This means that such products should provide a therapeutic benefit if consumed regularly within a diverse diet and with the condition of having the main nutrients extracted in a standardized manner and dosage [37][14].
Originally, cereal fermented beverages were mainly produced due to the need for conserving and utilizing various cereals and crops with affordable financial implications. Some of them, which are now commercially available as soft drinks and non-alcoholic beverages, were traditionally prepared as alcoholic beverages, having higher contents of alcohol [38][15].
Currently, “non-alcoholic” is a regulatory term and the laws regarding it vary across the globe. For example, EU regulation no. 1169/2011 simply states that a beverage must be labelled as an alcoholic drink if it has an alcoholic strength by volume (ABV) of over 1.2%. Moreover, in Great Britain, an alcohol-free drink has a maximum alcohol content of 0.05 % ABV; in Germany, a beverage is considered alcohol-free if the maximum alcohol limit is below 0.5 % ABV; in Spain, a non-alcoholic beer contains a maximum of 1 % ABV; in France, alcohol-free beers contain a maximum of 1.2 %; in US, non-alcoholic beers are of a maximum 0.5 % ABV; in China, non-alcoholic drinks can be of up to 0.5 % ABV [39][16]; in Japan, non-alcoholic beverages contain up to 1% ABV [40][17]. Depending on the country’s traditional recipe, boza, a lactic acid fermented drink, has an alcohol content of less than 1% ABV in Turkey and up to 7% ABV in Egypt [41][18], probably due to microorganisms involved in the fermentation process [42][19].
Fermented drinks were and are obtained using a combination of fermentable substrates, like cereal mixes, fruits, plants, spices, legumes, and vegetables. The possibility of combining various fermentable substrates and of adding supplementary bioactive compounds to the final product encourages the development of different versions of the same original recipes, according to specific resources, health concerns, and nutritional needs. Such enhancements can be seen, for example, in vitamin A-fortified mahewu [43][20] or in establishing the suitable temperature for saccharification and oligosaccharide production efficiency in amazake, containing up to 0.5 % ABV [44][21].
Globally, there are numerous similar non-alcoholic cereal fermented beverages with similar names and profiles targeted for thirst quenching properties, on nutrition added value, cultural significance, and on providing alternatives to alcoholic beverages. Fermented beverages based on cereals are somewhat common around the world as staple foods, particularly in developing countries and are all made in a similar manner, generally using spontaneous microbial cultures[15] [38]. Although there are various non-alcoholic cereal fermented beverages with different chemical profiles and sensory traits, all of them present certain bioactive compounds and therapeutic agents, which make them beneficial for human health. The functional compounds available and detected in NFCBs have variable values, therefore, it is difficult to assess their functional impact when consumed regularly [17][22]. If cereals lack certain nutrients, then additional food matrices can be added to enhance the final NFCB, as seen in a case of mahewu enhanced with Moringa oleifera leaf powder for elevating Ca and Fe contents [45][23]. Another example of a functional NFCB is a non-alcoholic beverage made of green tea and barley malt wort for delivering superior amino acid content [46][24].
In Table 1, there are several NFCBs listed with their place of origin, constituent cereals, microorganisms, nutritional compounds, and potential health benefits. As previously mentioned by other authors, the functional outcome of such products is strongly connected to the selection of the cereals, microorganisms, fermentation temperature and time, and other additional food matrices [47–49][25][26][27].
Table 1.
Non-alcoholic fermented cereal beverages and their microorganisms, nutritional composition, and health benefits.
|
Beverage/Place of Origin |
Cereals |
Microorganisms |
Nutritional Compounds |
Health Benefits |
Ref. |
|
Amazake–Japan |
rice koji |
Lactobacillus sakei, Aspergillus oryzae; |
amino acids; vitamins B1, B2, B6; pantothenic acid, vitamin E, flavonoids, dietary fibre, polysaccharides, sterols; |
improves digestion; mitigates hypertension, skin-enhancing action, alleviates liver cirrhosis (200 kcal/150 mL/day/ 12 weeks); |
|
|
Borș/Borsht–Central and Eastern Europe, Romania |
wheat bran, corn flour |
Lactobacillus delbrueckii ssp. Delbrueckii; |
lipophilic and hydrophilic antioxidants (tocopherols, tocotrienols), phenolic compounds, group B vitamins, vitamin E, alkylresorcinols; lignans; |
alleviates respiratory and digestive diseases (indigestion, vomiting), effective management of hepatic and bile diseases, potentially beneficial in cancer treatment; |
|
|
Boza–Turkey, Greece, Bulgaria, Albania, Romania, Bosnia Herzegovina; South Africa |
barley, oats, rye, millet, maize, wheat, rice |
Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus pentosus, Lactobacillus paracasei, Lactobacillus fermentum, Lactobacillus brevis, C. inconspicua, C. pararugosa; |
vitamin A, vitamins B1, B2, B6, nicotinamide; Ca, Fe, P, Zn, Na, β-glucan, dietary fibres; |
improves gastrointestinal health, stimulates the immune system, decreases cholesterol level; |
|
|
Busa–Syria, Egypt, Kenya, Turkistan; |
rice or millet |
Lactobacillus sp. Saccharomyces spp. |
dietary fibre, amino acids, fatty acids, vitamins B1, B2; |
lowers cholesterol and reduces risk of cancer and obesity, lowers blood pressure, beneficial in diabetes; |
|
|
Gowé–West Africa, Benin |
malted and non-malted sorghum, maize |
Lb. fermentum, Weissella confusa, Weissella kimchii, Lactobacillus mucosae, Pediococcus acidilactici, Pediococcus pentosaceus; |
amino acids (glutamic acid and leucine), minerals (Fe, Ca, Zn, P); |
certain lactic acid bacteria strains can help in preventing infections by urogenital pathogens; antimicrobial efficacy; |
|
|
Kunun-zaki–Nigeria |
wheat and sorghum /millet, wheat, malted rice |
Lb. plantarum, Lb. fermentum, Lactococcus lactis; Saccharomysces cerevisiae; |
minerals (Fe, Ca, Mg, K); |
provides micro- and macronutrients, improves nutritional status; |
|
|
Kvass–Lithuania, Russia, Eastern Poland |
extruded rye, malted barley |
Lactobacillus casei, Lb. sakei, P. pentosaceus, S. cerevisiae; |
vitamins B1, B3, B2, B6; dietary fibres, Zn, Cu, maltose, maltotriose, glucose, fructose; |
modulates metabolism, reduces flatulence, alleviates hyperacidity; |
|
|
Mahewu/Amahewu–Africa (Botswana, South Africa, Zimbabwe) |
maize, sorghum, millet malt or wheat flour |
Lb. brevis, L. casei, L. lactis, Lb. plantarum, S. cerevisiae, S. pombe; |
Na, K, Ca, Fe, Zn, Mn; dietary fibre, carbohydrates, group B vitamins; |
bacteriostatic and bactericidal properties against enteric pathogens; |
|
|
Munkoyo–Zambia, Democratic Republic of Congo |
maize |
Lb. plantarum, |
fibre, vitamins B1, B2, B3, B6, B12, Ca, Fe, Zn, proteins, crude fat, carbohydrates; |
suppresses diarrhoea; anti-allergen, antimicrobial properties; |
|
|
Obushera– Uganda |
sorghum flour or millet, maize |
L. Lactis, Lb. plantarum, Lb. fermentum, Lb. delbrueckii, Weissella confusa; |
proteins, minerals, fibre; |
NA |
[58] |
|
Oshikundu/Ontaku–Namibia |
pearl millet meal, sorghum, or pearl millet malt |
Lb. plantarum, L. lactis, Lb. delbrueckii ssp. delbrueckii, Lb. fermentum, Lb. pentosus, Lactobacillus curvatus; |
shikimic acid, maleic acid, phytic acid, succinic acid; vitamins B1, B2; Ca, Cu, Fe, K, Mg, Mn, Na, S, Zn, P; |
NA |
|
|
Pozol–South Eastern Mexico, Central America |
maize |
Lb. plantarum, Lb. fermentum, Lb. casei, Lb. delbrueckii, Leuconostoc sp., Bifidobacterium sp., Streptococcus sp., Saccharomyces sp.; |
group B vitamins; dietary fibre; |
reduces cholesterol levels; improves gastrointestinal health; bactericidal, bacteriolytic, bacteriostatic activities; |
|
|
Shalgam–Turkey |
bulgur flour (wheat) |
Lb. plantarum, Lb. paracasei, Lb. brevis Lb. fermentum, S. cerevisiae; |
β-carotene, group B vitamins, Ca, Na, Fe; |
antiseptic agent; probiotic food; regulates the digestive system’s pH; diuretic action; |
|
|
Tobwa (without malt, only LAB)/Togwa–East Africa, Tanzania, Zimbabwe |
maize, finger millet (togwa) |
Lb. plantarum, Lb. brevis, Lb. fermentum, Lactobacillus cellobiosus, P. pentosaceus, W. confusa; |
amino acids; dietary fibre; carbohydrates; vitamin B2, B9, B12; |
antimicrobial activity; enteropathogenic inhibition of Campylobacter jejuni and Escherichia coli; eases diarrhoea and prevents malnutrition; |
|
Beverage/Place of Origin |
Cereals |
Microorganisms |
Nutritional Compounds |
Health Benefits |
Ref. |
|
Amazake–Japan |
rice koji |
Lactobacillus sakei, Aspergillus oryzae; |
amino acids; vitamins B1, B2, B6; pantothenic acid, vitamin E, flavonoids, dietary fibre, polysaccharides, sterols; |
improves digestion; mitigates hypertension, skin-enhancing action, alleviates liver cirrhosis (200 kcal/150 mL/day/ 12 weeks); |
[50–53] |
|
Borș/Borsht–Central and Eastern Europe, Romania |
wheat bran, corn flour |
Lactobacillus delbrueckii ssp. Delbrueckii; |
lipophilic and hydrophilic antioxidants (tocopherols, tocotrienols), phenolic compounds, group B vitamins, vitamin E, alkylresorcinols; lignans; |
alleviates respiratory and digestive diseases (indigestion, vomiting), effective management of hepatic and bile diseases, potentially beneficial in cancer treatment; |
[54–56] |
|
Boza–Turkey, Greece, Bulgaria, Albania, Romania, Bosnia Herzegovina; South Africa |
barley, oats, rye, millet, maize, wheat, rice |
Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus pentosus, Lactobacillus paracasei, Lactobacillus fermentum, Lactobacillus brevis, C. inconspicua, C. pararugosa; |
vitamin A, vitamins B1, B2, B6, nicotinamide; Ca, Fe, P, Zn, Na, β-glucan, dietary fibres; |
improves gastrointestinal health, stimulates the immune system, decreases cholesterol level; |
[38,41,57–61] |
|
Busa–Syria, Egypt, Kenya, Turkistan; |
rice or millet |
Lactobacillus sp. Saccharomyces spp. |
dietary fibre, amino acids, fatty acids, vitamins B1, B2; |
lowers cholesterol and reduces risk of cancer and obesity, lowers blood pressure, beneficial in diabetes; |
[42,61] |
|
Gowé–West Africa, Benin |
malted and non-malted sorghum, maize |
Lb. fermentum, Weissella confusa, Weissella kimchii, Lactobacillus mucosae, Pediococcus acidilactici, Pediococcus pentosaceus; |
amino acids (glutamic acid and leucine), minerals (Fe, Ca, Zn, P); |
certain lactic acid bacteria strains can help in preventing infections by urogenital pathogens; antimicrobial efficacy; |
[62–66] |
|
Kunun-zaki–Nigeria |
wheat and sorghum /millet, wheat, malted rice |
Lb. plantarum, Lb. fermentum, Lactococcus lactis; Saccharomysces cerevisiae; |
minerals (Fe, Ca, Mg, K); |
provides micro- and macronutrients, improves nutritional status; |
[67–70] |
|
Kvass–Lithuania, Russia, Eastern Poland |
extruded rye, malted barley |
Lactobacillus casei, Lb. sakei, P. pentosaceus, S. cerevisiae; |
vitamins B1, B3, B2, B6; dietary fibres, Zn, Cu, maltose, maltotriose, glucose, fructose; |
modulates metabolism, reduces flatulence, alleviates hyperacidity; |
[17,71,72] |
|
Mahewu/Amahewu–Africa (Botswana, South Africa, Zimbabwe) |
maize, sorghum, millet malt or wheat flour |
Lb. brevis, L. casei, L. lactis, Lb. plantarum, S. cerevisiae, S. pombe; |
Na, K, Ca, Fe, Zn, Mn; dietary fibre, carbohydrates, group B vitamins; |
bacteriostatic and bactericidal properties against enteric pathogens; |
[73–76] |
|
Munkoyo–Zambia, Democratic Republic of Congo |
maize |
Lb. plantarum, |
fibre, vitamins B1, B2, B3, B6, B12, Ca, Fe, Zn, proteins, crude fat, carbohydrates; |
suppresses diarrhoea; anti-allergen, antimicrobial properties; |
[22,77–79] |
|
Obushera– Uganda |
sorghum flour or millet, maize |
L. Lactis, Lb. plantarum, Lb. fermentum, Lb. delbrueckii, Weissella confusa; |
proteins, minerals, fibre; |
NA |
[80] |
|
Oshikundu/Ontaku–Namibia |
pearl millet meal, sorghum, or pearl millet malt |
Lb. plantarum, L. lactis, Lb. delbrueckii ssp. delbrueckii, Lb. fermentum, Lb. pentosus, Lactobacillus curvatus; |
shikimic acid, maleic acid, phytic acid, succinic acid; vitamins B1, B2; Ca, Cu, Fe, K, Mg, Mn, Na, S, Zn, P; |
NA |
[81–83] |
|
Pozol–South Eastern Mexico, Central America |
maize |
Lb. plantarum, Lb. fermentum, Lb. casei, Lb. delbrueckii, Leuconostoc sp., Bifidobacterium sp., Streptococcus sp., Saccharomyces sp.; |
group B vitamins; dietary fibre; |
reduces cholesterol levels; improves gastrointestinal health; bactericidal, bacteriolytic, bacteriostatic activities; |
[38,84,85] |
|
Shalgam–Turkey |
bulgur flour (wheat) |
Lb. plantarum, Lb. paracasei, Lb. brevis Lb. fermentum, S. cerevisiae; |
β-carotene, group B vitamins, Ca, Na, Fe; |
antiseptic agent; probiotic food; regulates the digestive system’s pH; diuretic action; |
[59,86] |
|
Tobwa (without malt, only LAB)/Togwa–East Africa, Tanzania, Zimbabwe |
maize, finger millet (togwa) |
Lb. plantarum, Lb. brevis, Lb. fermentum, Lactobacillus cellobiosus, P. pentosaceus, W. confusa; |
amino acids; dietary fibre; carbohydrates; vitamin B2, B9, B12; |
antimicrobial activity; enteropathogenic inhibition of Campylobacter jejuni and Escherichia coli; eases diarrhoea and prevents malnutrition; |
[19,87–89] |
Some of the NFCBs presented in Table 1 are commercially available, with various scales of production, while others are mainly homemade beverages, produced for individual consumption using traditional methods. Busa, kunun-zaki, mahewu, munkoyo, obushera, oshikundu, pozol, and tobwa are produced in rural and urban areas and their production is essentially a home-based industry as for now, there is no large-scale factory production. Amazake is commercially available in Japan and it is classified as a soft drink [90,91][52][68]. Borş is also industrially produced and used as a flavour enhancer in Romanian gastronomy or it is consumed as a refreshing drink [55][33]. Boza is one of the most popular Bulgarian beverages and is industrially produced at a large scale in all countries of the Balkan Peninsula [92][69]. Kvass is a traditional fermented Slavic and Baltic beverage and is one of the most popular beverages in Russia, numerous varieties emerged due to its popularity and market demand. Currently, kvass production is designed according to traditional processes, with the implementation of modern biotechnological methods[50] [72]. Shalgam is a traditional beverage of southern Turkish cities and it is commercialized in various markets of European cities [86][70]. In Table 2, there are more details presented regarding NFCBs and their sensory properties, pH values, nature of use, fermentation status, and production scale.
Table 2.
Non-alcoholic fermented cereal beverages and their sensory properties, nature of use, and fermentation status.
|
Beverage |
Sensory Properties/pH |
Nature of Use |
The status of Fermentation/Production Scale |
Ref. |
|
Amazake |
cloudy appearance, sour-sweet taste; pH ~3.9; |
dessert, snack, natural sweetening agent, baby food, salad dressing; |
homemade and industrialised; |
|
|
Borș |
sour-bitter taste; odour notes: “bran”, “yogurt”, “goat milk-cheese”, “pungent/sour”, “ripe/fermented fruit”; pH 3.3–4.2; |
used as a souring ingredient in soups, nutritious drink; |
homemade and industrialised; |
|
|
Boza |
thick liquid, pale yellow colouring; sweet-sour taste; pH 2.93–3.72; |
nutritious food, snack; |
homemade and industrialised: |
|
|
Busa |
thick homogeneous suspension, light to dark beige; sweet-sour taste; pH 3.4–5.3; |
traditionally made and served as an alcoholic drink; |
homemade; |
|
|
Gowé |
brown/white colour; sweet, acidic, cereal taste; soft texture; pH ~3.5–4.7; |
thirst-quenching and energy drink; children’s food; |
traditional, small-scale processors; |
|
|
Kunun-zaki |
low viscosity, creamy appearance; sweet-sour taste; pH ~3.8; |
refreshing drink; nutritious beverage; |
homemade, local producers; |
|
|
Kvass |
slightly cloudy appearance, light-dark brown colour; sweet-sour taste; pH 3.2–4.3; |
soft drink; |
traditionally homemade; industrialised differently than the traditional approach; |
|
|
Mahewu/Amahewu |
creamy colour, sour taste; pH ~3.5; |
weaning food for infants, consumed in schools, farms, mines, etc. |
homemade, commercially produced in African countries; |
|
|
Munkoyo |
slight yellow colour; sweet, mildly sour taste; pH 3.3–4.2; |
consumed at household level; energy drink; |
homemade; |
|
|
Obushera |
moderately thick composition, pale brown colour; sweet and sour taste; pH <4.5; |
thirst quencher, social drink, energy drink, and weaning food; |
homemade; commercially relevant types: Obutoko, Obuteire, Ekitiribita; |
|
|
Oshikundu/Ontaku |
white colour, milky appearance, sweet taste; pH 3.3–3.7; |
a token of welcome and hospitality; consumed at special events and daily social interactions; |
homemade; local producers; |
|
|
Pozol |
yellow-brown colour; sweet-sour; slightly acidic taste; pH 3.8; |
food or refreshing beverage, consumed at religious ceremonies and for its curative properties; |
homemade in rural and urban areas of southeast Mexico; small- and large-scale producers; |
|
|
Shalgam |
red colour; sour taste; |
used as a medicine because of its antiseptic agents; |
home-scale level; |
|
|
Tobwa/Togwa |
opaque and brownish colour; sweet, occasionally sour taste; pH ~4; |
consumed as a popular energy source; |
industrially produced in Tanzania; |
|
Beverage |
Sensory Properties/pH |
Nature of Use |
The status of Fermentation/Production Scale |
Ref. |
|
Amazake |
cloudy appearance, sour-sweet taste; pH ~3.9; |
dessert, snack, natural sweetening agent, baby food, salad dressing; |
homemade and industrialised; |
[90,91,93] |
|
Borș |
sour-bitter taste; odour notes: “bran”, “yogurt”, “goat milk-cheese”, “pungent/sour”, “ripe/fermented fruit”; pH 3.3–4.2; |
used as a souring ingredient in soups, nutritious drink; |
homemade and industrialised; |
[54–56] |
|
Boza |
thick liquid, pale yellow colouring; sweet-sour taste; pH 2.93–3.72; |
nutritious food, snack; |
homemade and industrialised: |
[58–61,94] |
|
Busa |
thick homogeneous suspension, light to dark beige; sweet-sour taste; pH 3.4–5.3; |
traditionally made and served as an alcoholic drink; |
homemade; |
[42,95] |
|
Gowé |
brown/white colour; sweet, acidic, cereal taste; soft texture; pH ~3.5–4.7; |
thirst-quenching and energy drink; children’s food; |
traditional, small-scale processors; |
[65,66] |
|
Kunun-zaki |
low viscosity, creamy appearance; sweet-sour taste; pH ~3.8; |
refreshing drink; nutritious beverage; |
homemade, local producers; |
[67,68] |
|
Kvass |
slightly cloudy appearance, light-dark brown colour; sweet-sour taste; pH 3.2–4.3; |
soft drink; |
traditionally homemade; industrialised differently than the traditional approach; |
[71,72] |
|
Mahewu/Amahewu |
creamy colour, sour taste; pH ~3.5; |
weaning food for infants, consumed in schools, farms, mines, etc. |
homemade, commercially produced in African countries; |
[45,74–76] |
|
Munkoyo |
slight yellow colour; sweet, mildly sour taste; pH 3.3–4.2; |
consumed at household level; energy drink; |
homemade; |
[22,77–79] |
|
Obushera |
moderately thick composition, pale brown colour; sweet and sour taste; pH <4.5; |
thirst quencher, social drink, energy drink, and weaning food; |
homemade; commercially relevant types: Obutoko, Obuteire, Ekitiribita; |
[80,96] |
|
Oshikundu/Ontaku |
white colour, milky appearance, sweet taste; pH 3.3–3.7; |
a token of welcome and hospitality; consumed at special events and daily social interactions; |
homemade; local producers; |
[81–83] |
|
Pozol |
yellow-brown colour; sweet-sour; slightly acidic taste; pH 3.8; |
food or refreshing beverage, consumed at religious ceremonies and for its curative properties; |
homemade in rural and urban areas of southeast Mexico; small- and large-scale producers; |
[85,97] |
|
Shalgam |
red colour; sour taste; |
used as a medicine because of its antiseptic agents; |
home-scale level; |
[59,86] |
|
Tobwa/Togwa |
opaque and brownish colour; sweet, occasionally sour taste; pH ~4; |
consumed as a popular energy source; |
industrially produced in Tanzania; |
[87,88] |
3. Processing Technologies and their Outcome
The dietary attributes and sensory traits of cereal products can be at times viewed as inferior or deficient in comparison with those of milk and milk-based foods. Some of the reasons behind this include the smaller protein quantities, deficiency in certain amino acids (lysine), the presence of antinutrients (phytic acid, tannins, and polyphenols), and the coarse nature of grains [19][4].
The fermentation of starchy sources is more complex compared to that of low molecular sugars (glucose or sucrose) because, in general, the starch must at first be converted into fermentable sugars. To achieve an almost complete starch degradation, two main types of amylolytic enzymes are required (α-amylase and glucoamylase) [98][64].
Several methods have been engaged with the aim of enhancing the nutritional qualities of cereals. These include genetic improvement and amino acid supplementation with protein concentrates or other protein-rich sources, such as grain legumes or defatted oil seed meals of cereals. Additionally, several processing technologies, which include cooking, sprouting, milling, and fermentation, have been put into practice to improve the nutritional properties of cereals, although the best one is probably fermentation. In general, the spontaneous fermentation of cereals leads to a decrease in the level of carbohydrates, as well as some non-digestible poly- and oligosaccharides [35][79]. Certain amino acids may be synthesized, and the availability of B group vitamins may be improved [99][80]. Fermentation also provides optimum pH conditions for the enzymatic degradation of phytate, which is present in cereals in the form of complexes with polyvalent cations, such as iron, zinc, calcium, magnesium, and proteins. Such a reduction in phytate may result in a several fold increase in the amounts of soluble Fe, Zn, and Ca [19,100–102][4][81][82][83].
The current article advances one general processing technique, by compiling all the traditional recipes assessed and integrating germinated and non-germinated grains, to adapt it to an industrial scale with functional improvement. Several traditional processing steps can be applied in the production. The general outline of the process is essentially the same and that presented in Figure 1: the grains, after conditioning, are either soaked/wet milled or the grains are dry milled and the flour is extracted in water afterwards, the mix is boiled to gelatinize the starch, a source of enzymes to hydrolyse the gelatinized starch into fermentable sugars is added, and finally, spontaneous fermentation occurs [19,103][4][84].
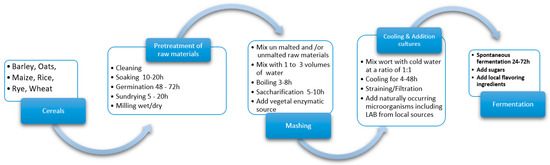
Figure 1. General flow diagram of traditional NFCB production.
3.1. Pre-Treatment of Raw Materials
Cereal processing is an essential component of the brewing production chain and the milling process is the main procedure. Before milling, the cleaning and conditioning of the grains is required. The cleaning process allows for the removal of various impurities, depending on the raw material types. The most common grain impurities are shrivelled grains, other cereals, grains damaged by pests, grains with discoloured germs, and sprouted grains. There are also miscellaneous impurities, including extraneous seeds, damaged grains, extraneous matter, husks, ergots, decayed grains, and insects. The conditioning or tempering of grains is performed using the monitored addition of water, which turns the endosperm softer and the bran harder. Doing this prevents the bran from breaking up, aids gradual separation throughout milling, and enhances sieving efficiency [104][85].
There are two milling categories, namely dry and wet milling, each having its own characteristics. Dry milling removes the germ and the outer fibrous materials of grains, as these by-products are not used in traditional ways [105][86]. Malting utilizes the power of natural germination when the grains, after absorbing water, germinate in the presence of oxygen to achieve a moisture content of up to 47% [106][87]. Another by-product is represented by floating kernels, which are unsuitable for malting. Throughout germination, the grain’s embryo expands, and rooting begins. Moreover, the germination and steeping processes frequently overlap. It is recommended to keep the germination time and temperature at low values, given that long and warm germination processes lead to longer roots, resulting in larger malt yield losses [104,106][85][87].
During germination, grain enzymes start to break down the endosperm high-molecular-weight material into easily digestible components for the yeasts. Drying the malt stops the germination process [104][85].
In the case of traditional methods, the grains are usually superficially cleaned. A part of the cleaned grains (in variable percentages) is washed and soaked (10 to 20 h) at room temperature (26–35° C). The soaked grains are drained and left for germination for 48 to 72 h with a frequent spraying of tap water. The germinated grains are sun dried (5 to 20 h), so the success of the process depends on the weather, mostly the intensity of the sunshine. Afterwards, the grains (malted and non-malted) are milled separately or in a mixture to obtain flour using rudimentary equipment [107][88].
Industrial cleaning processes aim at removing impurities and all other materials except for grains, using specific equipment such as magnetic separators, disc or sieve separators, aspirators, destoners, colour sorters, etc. The conditioning process ensures the complete hydration of grains, holding them in suitable containers for specific time intervals. Usually, depending on the grain varieties and initial moisture levels, the soaking time and temperature may be different [104][85].
For industrial-scale malting (Figure 2), the cereals are dried, and kilning is used to stop further transformations.
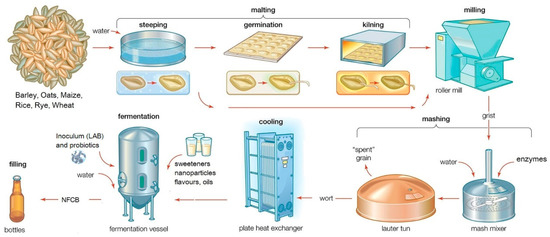
Figure 2.
General flow diagram of industrial NFCB production (compilation from Encyclopaedia Britannica’s brewing process).
During controlled drying, the water content should go under 5%, to stop the enzymatic activity whilst colour and flavour compounds are formed. A lot of by-products can additionally be recovered and further capitalized at an industrial scale. Dry milling can be extended to pearling, an abrasive procedure for gradually removing the testa and pericarp, aleurone and sub aleurone layers, and the germ. This results in polished grains and by-products with enhanced contents of bioactive compounds. Alternatively, wet milling, mostly used for producing starch and gluten, can increase the value of the cereals, as it is a source of by-products, with coproducts such as steep solids (abundant in compounds of pharmaceutical interest), germs (used in the oilseed-crushing industry), and bran [104][85].
3.2. Mashing
The traditional mashing process differs greatly, depending on the local culture, and has low efficiency. In Africa, either sorghum or finger millet malt is commonly used as a source of enzymes [22,108][10][89]. In the case of gowé production, a beverage based on maize and sorghum, the grains are milled to flour, which is optionally mixed/kneaded with tap or hot water or with a supernatant of a previous production and left to ferment at room temperature. Additionally, this processing technique can include a saccharification step, where a part of the malted sorghum or maize flour is kneaded with water and left for saccharification for 5 to 10 h [107][88]. For munkoyo, a maize-based beverage with a variable ABV, the specific feature is the usage of Rhynchosia roots or watery root extract as an enzymes source [22,77,78][10][90][91]. Starch gelatinization facilitates the activity of α- and β-amylases from the Rhynchosia roots for at least 4 h up to a maximum of 24 h, which hydrolyse the starch into fermentable sugars [22,103,109][10][84][37]. Mahewu is an example of a non-alcoholic sour beverage made from corn meal, consumed in Africa and some Arabian Gulf countries [74][53]. It is prepared from maize porridge further mixed with water. Sorghum, millet malt, or wheat flour is then added and left to ferment [19][4]. The production techniques of obtaining kvass, a cereal-based beverage produced from rye and barley malt, rye flour, and stale rye bread, uses as raw material either stale sourdough bread or malt rye malt and rye flour [110][92]. Boza is a colloid suspension, non-alcoholic beverage consumed in Bulgaria, Albania, Turkey, and Romania, made from wheat, rye, millet, maize, and other cereals mixed with sugar or saccharine [92][69]. Boza’s preparation involves six stages: the preparation of raw materials, boiling, cooling, straining, the addition of sugar, and fermentation. Another option for its production is the use of previously fermented boza as an inoculum [59][93].
The industrial mashing process resembles the beer production process. Between one and three volumes of water are added per volume of milled grains, and during the cooking process, the mixture turns into a mash. The mixture is cooked at a normal pressure or in an autoclave for about 2 h at 4–5 atmospheres.
The macromolecular profile of cereal-based beverages is generally determined by polymeric compounds (proteins, polysaccharides, and polyphenols) and their progress in depolymerization during processing [111,112][63][94]. Given that yeasts or specific strains of lactic acid bacteria (LAB) cannot metabolize high-molar-mass substances [113][95], the macromolecules are solely depolymerized during the malting and mashing process by the malt’s intrinsic enzymes. During malting, the enzymatic degradation of polymers is technologically controlled by the degree of steeping, germination time, and germination temperature [114,115][96][97]. Modern brewing barley varieties are bred to be balanced in malting performance and to meet the required brewing specifications. The degradation of starch into fermentable sugars (amylolysis) is the primary objective of mashing (substrate production for fermentation) [116][98], since during the subsequent fermentation process, only low-molar-mass compounds, fermentable sugars, and low-molar-mass proteinaceous compounds are metabolized by microorganisms (e.g., LAB and yeasts) [117,118][99][100].
Lautering is the next step in the large-scale process, through which solid and liquid fractions, respectively, the spent grain, composed of sugar-extracted grist or solids remaining in the mash, and the sweet wort with high contents of fermentable sugars are obtained. The spent grain is the major by-product of the brewing industry and represents a valuable source of bioactive ingredients and a potential ingredient for functional foods [119][101]. In small-scale production, malt extract can be used instead, thus skipping the use of grain malt (including milling, mashing, and lautering) [120][102].
3.3. Cooling and Addition of Yeast, LAB Cultures, and Other Ingredients
After boiling, the mash is gradually mixed with cold water in a 1:1 ratio. This slurry is often filtered or decanted to remove the grinding waste and insoluble plant material. In many traditional processes, where cereals are soaked in water for a few days, a succession of naturally occurring microorganisms will result in a population dominated by LAB. In such types of fermentations of endogenous grains, amylases generate fermentable sugars, which serve as energy sources for lactic acid bacteria. When no malted cereals are used, sucrose is added to the beverage to mimic the malt’s sweet taste.
The bacterial flora formed in each fermented cereal drink is influenced by several factors, such as water activity, pH level, salt concentration, temperature, and the composition of the grain matrix, which must be considered in industrial processes. However, most fermented drinks, including the well-known products commonly met in the Western world, as well as those beverages of other origins which are less well studied and characterised, rely on lactic acid bacteria to mediate the fermentation process [19,121][4][103]. Lactic acid fermentation contributes towards the nutritional value, shelf life, safety status, and acceptability of a wide range of cereal-based foods [118][100]. Fermentation is often just one step in the process of fermented food preparation. Other operations, such as volume reduction, salting, or heating, also affect the final product characteristics [19,122][4][104]. Depending on the desired product, further steps can be applied afterwards, such as standardisations and the addition of other ingredients like flavourings, sugar, and stabilizers.
3.4. Fermentation Process
Despite the lack of process control, dealing with unstandardised microbial flora composition, delayed fermentation, and imperfect reproducibility of the fermentation process, spontaneous fermentation offers complex microbial diversity, providing higher levels of intrinsic stability to the microbial community [123][105]. This is achievable due to stabilizing interactions between species that prevent and inhibit the proliferation of unwanted microorganisms, including pathogenic ones. Recently, the existence of stability criteria for complex microbial communities was proven [124][106]. In terms of spontaneous fermentation, this can be explained by the resilience to small perturbations when there is a balance between the availability of resources, namely nutrients, and consumers (e.g., lactic acid bacteria). Commonly, the production of artisanal fermented beverages is conducted in successive batches [55][33], by using a natural starter from the previous fermentation. At all traditional sites, spontaneous fermentation proceeds after cooling. A 24 h fermentation period is sufficient for some traditional beverages to develop their characteristic sensory attributes, although in practice, fermentation time can go on for up to three days. Some brews, obtained through a 6 to 15 h fermentation interval of non-malted grain flour, are enhanced with commercial sugar (sucrose) to obtain the sweet taste. This process is a distortion of the original germination technique for gowé production [22][10]. A modern industrial process is different from the traditional process regarding the introduction and control of thermophilic LAB cultures, which only produce lactic acid, the extension of the product’s shelf life by pasteurization and/or chemical preservation, and the inclusion of sugar and/or artificial sweeteners [125,126][107][108]. Controlled fermentation also leads to a general improvement in the shelf life, texture, taste, and aroma of the final product. During cereal fermentation, several volatile compounds are formed, which contribute to a complex blend of flavours [127][109]. Moreover, there is a good opportunity to apply colloidal dispersions in the form of nanoemulsions to deliver food grade nanoparticles, which contain water-insoluble molecules that were formerly unsuitable due to their poor soluble characteristics. Thus, there is a wide range of healthy foods which can be further designed, such as cereal-based fermented beverages enhanced with nanomolecules possessing beneficial health attributes [27][110].
4. Fermentation Microbiota and Safety of NFCBs
Originally, fermented beverages were only consumed in their native regions, however, due to increasing demand and interest, some of those traditional beverages are available to international markets. The attributes of traditional fermented beverages are influenced by several factors, such as the use of different raw materials, manufacturing methods, natural microbiota, and fermentation conditions. The microbiology of many traditional fermented drinks prepared from the most common types of cereals is quite complex.
There is a lot of diversity in the traditional processing techniques used for cereal-based fermented beverages all over the world, integrating single or multigrain cereals, germinated and/or non-germinated grains. Many types of cereal-based fermented beverages are produced in Africa, such as togwa in Tanzania, mahewu in Zimbabwe and South Africa, mawè in Benin, and munkoyo in Zambia and the Democratic Republic of the Congo [9,19,128,129][9][4][111][112]. Generally, the preparation of these products is a traditional family activity with an uncontrolled fermentation process by diverse microbial communities. The composition of the microbial community in a fermented food product largely determines the key product properties [130,131][113][114]. In other words, variations in microbial communities may result in differences in product quality, taste, acceptability, and microbial stability.
In one of our previous papers [55][33], we proved the impact of processing parameters, namely temperature and batch fermentation cycles, on the chemical composition of borș. Interestingly, the final composition of cereal-based beverages might not only be influenced by the physical process parameters. Processing practice variation affects the microbial composition of the fermenting microbial community. Despite the decreased pH caused by the lactic acid bacteria activity, the low pH of munkoyo also permitted the development of acidifying bacteria [22][10]. An important role is played by the initial microbial composition of the raw materials used in the process. The same study also referred to the fact that further investigations might be needed into the soil composition of the harvested raw materials. Along with the prevention of growth of most pathogenic strains [132][115], a low pH (of 2.5 to 3.5) improves food safety and expands the shelf life of this type of beverage [22][10]. The pathogenic microorganisms that are aerobes and facultative anaerobes and ferment simple sugars have an optimum pH for growth of 6.0 to 8.0. However, growth can occur at a pH as low as 4.3 and as high as 9, but with a combination of factors (pH, water activity), the control of foodborne pathogen growth can be done [133][116].
The fermentation of most cereals is natural and involves mixed cultures of yeasts, bacteria, and fungi [134][117]. Some microorganisms may participate in parallel, while others act in a sequential manner with a changing dominant flora during fermentation. The challenge, though, is the generally uncontrolled nature of the fermentation, which raises safety concerns, as well as the lack of standardization in the methods used, thus further research and development are needed to improve the traditional fermentation processes [80][58]. In this regard, the introduction of starter culture technology has led to greater consistency and safety and to better product quality[118] [135].
Furthermore, the main difficulties encountered in the production of cereal-based beverages using traditional processes are linked to the high variability of unit operations and the unhygienic conditions of the processing environment. The soaking and germination parameters (temperature, duration, moisture) vary within and between processors. Moreover, during soaking and germination, the grains can be infested by fungi with the potential development of mycotoxins (aflatoxins) [136][119].
The continuous study of the fungi, yeasts, and bacteria strongly involved in ensuring a certain quality of the NFCB allows for the optimization of same-product delivery[120][62][121] [85,137,138]. Such upgraded fermented beverages are sometimes the outcome of general efforts to enhance original recipes [139][122]. Kunun-zaki is an example of a traditional wheat and sorghum fermented beverage now also commercially available in the form of a powder [68][46], and there are several strategies proposed to upgrade and re-engineer the process of gowé production, a beverage obtained from sorghum and maize[44] [66]. As seen so far, it is possible to prolong shelf life through the co-incubation of probiotic cultures, as seen in the development of some cereal-based fermented beverages[14][123] [37,140] or to market to a group of consumers looking for healthy and functional foods by using oats, for example, as a main ingredient [141][124]. In-depth studies are still being conducted to ensure food safety in the processing technology of fermented foods through the keen selection of starter cultures and thorough examination of the specific microorganisms [142,143][125][126]. For example, it was recently shown that Enterococcus faecium YT52 isolated from boza is susceptible to clinically relevant antibiotics and contains low numbers of virulence factors and antibiotic resistance genes. Therefore, the enterocin-producing E. faecium YT52 strain poses a low risk to consumer health, and this strain may be used as a starter or a co-starter culture for improving the food safety of fermented products by acting against foodborne pathogens, such as Listeria monocytogenes and Bacillus cereus [144][127]. Moreover, rethinking the technological processes for obtaining various cereal-based fermented beverages helps to increase their functionality and overall therapeutic and nutritional properties. Such an example is boza, enhanced by fermenting cereals with Lactobacillus acidophilus, Bifidobacterium bifidum, and Saccharomyces boulardii, and by adding chickpea flour to the fermentable substrate for an elevated protein content [145][128]. Along with these interventions, narrowing the risks of product spoilage through the inoculation of specific strains of LAB and yeasts and processing the cereals in a certain manner repetitively allows the producers to deliver same-quality functional NFCBs.
Fermentation is one of the oldest known biotechnologies and the most economical procedure for producing new foods and ensuring product conservation. The yeasts responsible for fermentation in fermented drinks include species of Saccharomyces, Saccharomycopsis, Schizosaccharomyces, Pichia, Candida, Torulopsis, and Zygosaccharomyces. Brewer’s yeast, Saccharomyces cerevisiae, metabolizes various sugars, mainly into alcohol, but also into other flavour-active substances. The most used microorganisms in fermentation processes belong to Lactobacillus species, which synthesize many flavour-active substances and lactic acid and are considered probiotic microorganisms known to support intestinal microbiota [146][129]. Fermented beverages are known to be rich in bioactive compounds, such as immune globulin peptides and the bioactive hormone cytokinin [19,71,82,147,148][4][49][38][130][131]. The use of a wide variety of microorganisms and yeasts is implied in the production of NFCBs by both traditional and modern means.
Largely responsible for the fermentation process are the indigenous microbiota present on the substrate or which can be added artificially as a culture. Basically, there are four main fermentation processes, which include alcohol production, lactic acid development, acetic acid production, and alkaline fermentation [19][4]. The modern industry for fermentative foods and beverages is innovative, given that it currently employs thermophilic fermentation, DNA technologies, molecular devices, designed starter cultures, genetic engineering, etc. Recombinant DNA technology has provided new insights for enhancing the product quality by designing tailor-made starter cultures that perform better than those found naturally [60][132]. For example, Basinskiene et al. used Lactobacillus sakei KTU05-6 and Pediococcus pentosaceus KTU05-10 to ferment extruded rye for obtaining kvass, a traditional Lithuanian NFCB. They showed that the pH of the beverages fermented by LAB reached lower values compared to yeast fermentation and a they had a higher amount of organic acids. Innovative technology was applied, such as xylanolytic enzymes and antimicrobial active LAB, to improve the product’s functional properties [71][49].
Functional cereal fermented beverages are becoming more attractive as they represent healthy alternatives for lactose intolerant consumers and for those who avoid certain allergens. For example, a study describes the production of kefir-like riboflavin-enriched beverages based on oat, maize, and barley flours. To obtain this beverage, riboflavin-producing Andean LAB were used, consisting of five Lactobacillus plantarum strains and two Leuconostoc mesenteroides strains [149][74].
As previously discussed, ensuring product safety, although difficult at times, is a crucial step in the production of traditional beverages where the fermentation process is spontaneous. Thus, microorganisms found on the brewers’ skin, hair, and clothes can alter the product safety, therefore, high standards of hygiene are mandatory. Nevertheless, through the identification of bacteria strains and yeasts, the safety and quality status of the fermented beverages are guaranteed. A safety case study was conducted concerning “obushera”, a Ugandan traditional fermented cereal beverage where important steps, such as pasteurization and ensuring water quality, are in the loop to ensure product safety and quality, as pathogens can also change the product’s sensory characteristics [80][58].
Superior results can be obtained in the production of NFCBs by better understanding the interaction of microorganisms in the fermented substrates. The beneficial outcomes of controlling microorganisms are mirrored in product safety, increased shelf life, improved nutrient contents and availability, palatability, and enhanced sensory traits. For example, it was shown that S. cerevisiae improves LAB growth by transmitting essential metabolites, such as pyruvate, amino acids, and vitamins, while it uses some metabolites produced by LAB as carbon sources [150][133]. Moreover, Salari et al. concluded in their study that malt and L. delbrueckii were the best substrate and lactic strain for producing a functional beverage with the highest cell viability (1.2×106 cfu/mL after 4 weeks) [151][134].
Probiotics
Non-alcoholic fermented cereal-based beverages contain a wide range of diverse probiotics, depending on their cereal substrate and overall production methods. The processing method should assure the stability of the bacterial composition in order for the final product to possess probiotic functionality [152][135]. Still, the threshold to declare a beverage a probiotic one must be higher than 107 CFU/mL. Moreover, not all lactic acid bacteria possess probiotic activity. L. rhamnosus, also present in NFCBs (Table 1), was efficient in treatment and prevention of gastrointestinal disease [152][135]. The traditional Romanian NFCB, borș, has been consumed since ancient times as a gastric remedy. Several potentially probiotic bacteria—L. casei, L. plantarum, L. brevis, and L. fermentum—were isolated from borș, explaining the traditional consumption of this beverage for curative purposes [56][34].
The predominant microorganisms in the spontaneous fermentation of the African mahewu, a non-alcoholic sour beverage made from corn meal and consumed in Africa and some Arabian Gulf countries, belong to Lactococcus lactis subsp. lactis. On the other hand, the microbiota identification of Bulgarian boza shows that it mainly consists of lactic acid bacteria and yeasts, such as Lactobacillus plantarum, L. acidophilus, L. fermentum, L. coprophilus, Leuconostoc raffinolactis, Ln. mesenteroides, and Ln. brevis and Saccharomyces cerevisiae, Candida tropicalis, C. glabrata, Geotrichum penicillatum, and G. candidum, respectively [19][4].
In the case of Turkish boza, the use of LAB and yeast isolates as starter cultures allows for controlled fermentation studies to be carried out. The selection of proper strains with probiotic and antimicrobial properties enhances the functional properties of boza [59][93].
As seen in the case of African countries, the primary challenge for the development and use of fermented cereal-based probiotic beverages is the common lack of knowledge regarding the health and nutritional benefits of such foods and beverages. Insufficient common knowledge on probiotics and their benefits creates a sense of scepticism among consumers. Moreover, there is also an imperative need to ensure proper facilities for probiotic starter cultures, given that through spontaneous fermentation, the organoleptic and functional qualities of the resulting products are variable [153][136].
5. The Nutritional and Bioactive Composition of Commonly Consumed NFCBs
Consumers are aware of the importance of maintaining a strong immune system to prevent illnesses and they are actively looking for products which can help maintain their health status and alleviate health problems. It has been scientifically proven that probiotics isolated from functional beverages boost the immune system [154][137], and that, along with prebiotics, they are able to improve the intestinal homeostasis, immunomodulating ability, and general health of the host [27][110].
Looking at past publications, it is shown how fermented beverages have transitioned from traditional natural fermented products to beverages formulated with functional ingredients meant to offer cardiovascular health benefits, and then to functional fermented drinks which improve gastrointestinal health, which could then evolve into fermented products containing specific bioactive nanoparticles [134][117].
NFCBs are receiving increased attention from researchers and consumers more recently due to their proven probiotic characteristics and disease prevention perspectives [17][22]. The perceived health outcomes of fermented beverages are strongly related to the microbial content and implicit improvement of gastrointestinal health [38][15]. Moreover, non-alcoholic fermented beverages offer a sense of wellbeing, as they stimulate the metabolic system [126][108].
Fermented cereal-based foods, including NFCBs, are a potential source of new functional lactic acid bacteria species besides various nutrients and bioactive compounds, with beneficial effects on human health [56][34]. Furthermore, as briefly mentioned previously, NFCBs are healthy alternatives to the traditionally consumed food probiotics of dairy origin (e.g., yogurt, kefir, etc.), especially for people with lactose intolerance and milk protein allergies [27][110].
Given the functional components of fermented beverages and their bioactive compounds released through fermentation by cultures, NFCBs have been linked with many potential and some proven health benefits and actions on digestive, endocrine, cardiovascular, immune, and nervous levels [155][138]. They present beneficial actions for vital body functions and contribute to the prevention and reduction of risk factors for various diseases [19,23][4][139]. NFCBs are rich sources of minerals, vitamins, fibre, flavonoids, phenolic compounds, antioxidants, omega-3 fatty acids, plant extracts, sterols/stanols, amino acids, and biopeptides, among others, which could also protect from oxidative stress and inflammation diseases [17,134][22][117]. The presence of numerous valuable compounds in NFCBs grants several health benefits upon consumption, as presented in Figure 3.
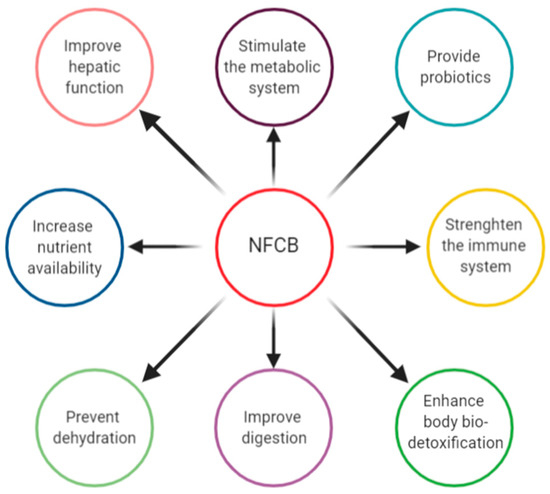
Figure 3. Health benefits associated with NFCB consumption.
Considering group B vitamins, these are unequally distributed in grain tissues. What counts the most in terms of their potential functionality in cereal-based food and beverages is their biological availability. During thermal food processing, these vitamins are destroyed almost completely, however, lactic acid fermentation represents a great tool for food industrialists interested in developing novel vitamin-fortified products. For example, Capozzi et al. (2012) pointed out the ability of many lactic acid bacteria strains, such as Lactobacillus delbrueckii, Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus fermentum, and Pediococcus lactis, reported in the composition of several NFCBs (Table 1), to biosynthesize riboflavin [156][140].
A new concept attributed to non-alcoholic cereal-based beverages is that they are healthy drinks with important impacts on human health. It has been shown that consuming NFCBs leads to liver function improvement, increased levels of lactobacilli and bifidobacteria in the intestinal microbiota [157][141], balanced gut microbiota, and the prevention of bacterial translocation with reduced incidences of nosocomial infections[1] [27]. Furthermore, because non-alcoholic fermented beverages contain several LAB metabolites, their consumption confers bactericidal, bacteriolytic, and bacteriostatic properties, resulting in therapeutic effects at a digestive level. Antimicrobial compounds identified in functional beverages exhibited activities against several Gram-positive and Gram-negative bacteria and against yeasts and moulds [17][22]. The functional compounds and potential or proven health benefits of NFCBs are presented in Table 1.
As previously discussed, the potential benefits of NFCBs can be ensured in a safe and non-contaminated production environment to prevent the risk of product spoilage. Moreover, there are diverse nutritional and bioactive components of NFCBs, depending on the cereal substrate, microorganisms, and fermentation parameters, and on the technological process followed.
Currently, novel NFCBs are designed based on traditional recipes for delivering improved functional fermented beverages. Such an example is “amahewu” or “mahewu”, a southern African LAB-fermented non-alcoholic maize-based beverage, which is deficient in vitamin A. In a study published in 2015, the traditional drink was redesigned using provitamin A-biofortified maize in exchange for the traditionally used white maize and it resulted in a functional beverage addressing vitamin A deficiency, a concerning health problem in sub-Saharan Africa [43][20].
5.1. Phenolic Compounds and Antioxidant Activity
Cereal grains and germs contain various major phytochemicals, such as phenolic acids, flavones, phytic acid, flavonoids, coumarins, and terpenes. The bran layers of cereal grains are relatively rich in water soluble and liposoluble antioxidants. Oats include phenolic acids, flavonoids, tocopherols, tocotrienols, and avenanthramides. On the other hand, insoluble fibre of wheat bran contains about 0.5%–1% phenolics and most phenolic compounds, like ferulic acid, are abundant in whole grains [158][142].
Depending on the cereal substrate, fermentation type, and other added ingredients, each NFCB can have quite different compositions. For example, the phenolic profile of brown beer vinegar indicates that such a fermented beverage is a rich source of phenolic compounds (661 mg GAE/L) and antioxidants, with 30% increased antioxidant activity after acetic fermentation. The same trend was observed regarding some of the drink’s individual phenolic compounds. With cohumulone I (4.44 mg CE/L), cohumulone II (6.58 mg/L), 8-prenylnaringenin (2.33 mg CE/L), 6- prenylnaringenin (1.86 CE/L), humulone (5.62 CE/L), and isohumulone (4.14 CE/L) [159][143], this non-alcoholic fermented beverage can be considered an alternative source of valuable compounds, which could also be part of specific diets.
Fermentation parameters are of high importance in what concerns an NFCB’s antioxidant profile. For example, a fermentation temperature of 24° C was considered a positive factor influencing the antioxidant activity of borș, a Romanian traditional wheat bran-based fermented beverage [55][33]. The antioxidant activity ranged from 3.05 to 8.52 mmol/L Trolox.
Some of the ancient traditional fermented beverages are currently produced at industrial scale. Still, the original recipes and the nutritional components are being altered because of thermal treatments applied to obtain product safety and stability. The addition of natural starters allowed for the increase in phenolic contents and the enhancement of final antioxidant activity. The same trend considering phenolic content between fermentation stages was found in the case of borș [56][34]. Depending on the processing operations applied, the phenolic compounds ranged considerably (4-hydroxybenzoic acid: 0.9–5.9 mg/L; vanillic acid: 0.6–3.2 mg/L; syringic acid: 0.5–2.5 mg/L; p–coumaric acid: 0.5–1.5 mg/L; sinapic acid: 0.6–2.7 mg/L; ferulic acid: 13.7–47.8 mg/L).
5.2. Amino Acids
Although cereals pose few challenges from a nutritional standpoint, especially with regard to the increased starch content upon cooking, the limited amino acid contents of their protein fractions, or the reduced bioavailability of their zinc and iron contents, there are already some solutions meant to correct and further enhance the nutritional status of cereal-based foods and beverages [160][144].
For example, in a study conducted on borș, an increase in amino acid content was found from one fermentation stage to another. The same study stated that the LAB fermentation of cereals improves the protein quality as well as the level of certain free amino acids by enhanced endogenous proteolysis and/or microbial action. Among the identified amino acids, there were isoleucine and threonine [56][34].
In general, the natural fermentation of cereals allows for amino acids to be synthesised, while it also helps to enhance the availability of group B vitamins. Fermentation processes also offer suitable pH conditions for phytate enzymatic degradation. Such a reduction in phytate may increase the amount of soluble iron, zinc, and calcium several fold, correcting the nutritional status of cereal-based beverages and foods [19][4].
References
- Salmerón, I. Fermented cereal beverages: From probiotic, prebiotic and synbiotic towards Nanoscience designed healthy drinks. Lett. Appl. Microbiol. 2017, 65, 114–124.
- Schwan, R.F.; Ramos, C.L. Functional Beverages from Cereals. In Functional and Medicinal Beverages; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128163979
- Adriana Păucean; Simona Maria Man; Maria Simona Chiş; Mureşan; Carmen Rodica Pop; Sonia Ancuţa Socaci; Sevastita Muste; Man; Chiş; Pop; et al.Crina Carmen MureşanCrina C. MureșanVlad MureşanCarmen Pop Use of Pseudocereals Preferment Made with Aromatic Yeast Strains for Enhancing Wheat Bread Quality. Foods 2019, 8, 443, 10.3390/foods8100443.
- A. Blandino; M.E. Al-Aseeri; S.S Pandiella; D. Cantero; Colin Webb; Cereal-based fermented foods and beverages. Food Research International 2003, 36, 527-543, 10.1016/s0963-9969(03)00009-7.
- Valery Ripari; Techno-Functional Role of Exopolysaccharides in Cereal-Based, Yogurt-Like Beverages. Beverages 2019, 5, 16, 10.3390/beverages5010016.
- Călinoiu, L.F.; Vodnar, D.C. Whole grains and phenolic acids: A review on bioactivity, functionality, health benefits and bioavailability. Nutrients 2018, 10, 1615.
- Bernardo, C.O.; Ascheri, J.L.R.; Carvalho, C.W.P.; Chávez, D.W.H.; Martins, I.B.A.; Deliza, R.; de Freitas, D.G.C.; Queiroz, V.A.V. Impact of extruded sorghum genotypes on the rehydration and sensory properties of soluble beverages and the Brazilian consumers’ perception of sorghum and cereal beverage using word association. J. Cereal Sci. 2019, 89, 102793.
- Munekata, P.E.S.; Domínguez, R.; Budaraju, S.; Roselló-Soto, E.; Barba, F.J.; Mallikarjunan, K.; Roohinejad, S.; Lorenzo, J.M. Effect of innovative food processing technologies on the physicochemical and nutritional properties and quality of non-dairy plant-based beverages. Foods 2020, 9, 228
- Cheryl G. Fernandesa; Sachin K Sonawaneb; Arya S.S.; CEREAL BASED FUNCTIONAL BEVERAGES: A REVIEW. Journal of Microbiology, Biotechnology and Food Sciences 2018, 8, 914-919, 10.15414/jmbfs.2018-19.8.3.914-919.
- Sydney Phiri; Sijmen E. Schoustra; Joost Van Den Heuvel; Eddy J. Smid; John Shindano; Anita R Linnemann; Fermented cereal-based Munkoyo beverage: Processing practices, microbial diversity and aroma compounds. PLoS ONE 2019, 14, e0223501, 10.1371/journal.pone.0223501.
- Yu, H.; Bogue, J. Concept optimisation of fermented functional cereal beverages. Br. Food J. 2013, 115, 541–563.
- Nyanzi, R.; Jooste, P.J. Cereal-Based Functional Foods, Probiotics, Everlon Cid Rigobelo. IntechOpen 2012, 161–197
- Daniel Granato; Domingos Sávio Nunes; Francisco J. Barba; An integrated strategy between food chemistry, biology, nutrition, pharmacology, and statistics in the development of functional foods: A proposal. Trends in Food Science & Technology 2017, 62, 13-22, 10.1016/j.tifs.2016.12.010.
- Daniel Granato; Francisco J. Barba; Danijela Bursać Kovačević; José M. Lorenzo; Adriano G. Cruz; Predrag Putnik; Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annual Review of Food Science and Technology 2020, 11, 93-118, 10.1146/annurev-food-032519-051708.
- Alan J. Marsh; Colin Hill; R. Paul Ross; Paul D. Cotter; Fermented beverages with health-promoting potential: Past and future perspectives. Trends in Food Science & Technology 2014, 38, 113-124, 10.1016/j.tifs.2014.05.002.
- Magdalena Müller; Konstantin Bellut; Johannes Tippmann; Thomas Becker; Physical Methods for Dealcoholization of Beverage Matrices and their Impact on Quality Attributes. ChemBioEng Reviews 2017, 4, 310-326, 10.1002/cben.201700010.
- Motoyoshi Kubo; Yuji Nozu; Chie Kataoka; Masako Kudo; Shiori Taniguchi; Yuki Sato; Naoko Nakayama; Motoi Watanabe; Correlation between Non-Alcoholic Beverage Consumption and Alcohol Drinking Behavior among Japanese Youths. Open Journal of Preventive Medicine 2015, 5, 31-37, 10.4236/ojpm.2015.52004.
- Muhammet Arici; Orhan Dağlıoğlu; Boza: A lactic acid fermented cereal beverage as a traditional Turkish food. Food Reviews International 2002, 18, 39-48, 10.1081/fri-120003416.
- Shonisani Eugenia Ramashia; Tonna Ashim Anyasi; Eastonce Tend Gwata; Stephen Meddows-Taylor; Afam Israel Obiefuna Jideani; Processing, nutritional composition and health benefits of finger millet in sub-saharan Africa. Food Science and Technology 2019, 39, 253-266, 10.1590/fst.25017.
- Temitope D. Awobusuyi; Muthulisi Siwela; Unathi Kolanisi; Eric O. Amonsou; Provitamin A retention and sensory acceptability of amahewu, a non-alcoholic cereal-based beverage made with provitamin A-biofortified maize. Journal of the Science of Food and Agriculture 2015, 96, 1356-1361, 10.1002/jsfa.7230.
- Yoshifumi Oguro; Ayana Nakamura; Atsushi Kurahashi; Effect of temperature on saccharification and oligosaccharide production efficiency in koji amazake. Journal of Bioscience and Bioengineering 2019, 127, 570-574, 10.1016/j.jbiosc.2018.10.007.
- Aristea Baschali; Effie Tsakalidou; Adamantini Kyriacou; Nena Karavasiloglou; Antonia-Leda Matalas; Traditional low-alcoholic and non-alcoholic fermented beverages consumed in European countries: a neglected food group. Nutrition Research Reviews 2017, 30, 1-24, 10.1017/s0954422416000202.
- R.N. Olusanya; U. Kolanisi; A. Van Onselen; N.Z. Ngobese; M. Siwela; Nutritional composition and consumer acceptability of Moringa oleifera leaf powder (MOLP)-supplemented mahewu. South African Journal of Botany 2020, 129, 175-180, 10.1016/j.sajb.2019.04.022.
- Gernet M. V; Gribkova I. N.; Kobelev K. V; Nurmukhanbetova Dinara Erikovna; Assembayeva Elmira Kuandykovna; BIOTECHNOLOGICAL ASPECTS OF FERMENTED DRINKS PRODUCTION ON VEGETABLE RAW MATERIALS. NEWS of National Academy of Sciences of the Republic of Kazakhstan 2019, 1, 223-230, 10.32014/2019.2518-170x.27.
- Fallourd, M.J.; Viscione, L. Ingredient Selection for Stabilisation and Texture Optimisation of Functional Beverages and the Inclusion of Dietary Fibre; Woodhead Publishing Limited: Cambridge, UK; Sawston, UK, 2009; ISBN 9781845693428.
- Vinicius De Melo Pereira, G.; De Carvalho Neto, D.P.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.J.; Magalhães Júnior, A.I.; Soccol, C.R. A Review of Selection Criteria for Starter Culture Development in the Food Fermentation Industry. Food Rev. Int. 2020, 36, 135–167.
- Pontonio, E.; Rizzello, C.G. Minor and Ancient Cereals: Exploitation of the Nutritional Potential Through the Use of Selected Starters and Sourdough Fermentation, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128146392
- Shin Oura; Sachiko Suzuki; Y Hata; Akitsugu Kawato; Yasuhisa Abe; Evaluation of physiological functionalities of amazake in mice.. JOURNAL OF THE BREWING SOCIETY OF JAPAN 2007, 102, 781-788, 10.6013/jbrewsocjapan1988.102.781.
- Yumiko Nagao; Effect of a Late Evening Snack of Amazake in Patients with Liver Cirrhosis: A Pilot Study. Journal of Nutrition & Food Sciences 2013, 3, null, 10.4172/2155-9600.1000223.
- Alauddin, M.; Kabir, Y. Functional and Molecular Role of Processed-Beverages toward Healthier Lifestyle. In Nutrients in Beverages; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128168424
- Hiroko Maruki-Uchida; Masahiko Sai; Shoichiro Yano; Minoru Morita; Kazuhisa Maeda; Amazake made from sake cake and rice koji suppresses sebum content in differentiated hamster sebocytes and improves skin properties in humans. Bioscience, Biotechnology, and Biochemistry 2020, null, 1-7, 10.1080/09168451.2020.1756734.
- Nicolau, A.I.; Gostin, A.I. Safety of Borsh; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128006207
- Antonella Pasqualone; Carmine Summo; Barbara Laddomada; Elena Mudura; Teodora Coldea; Effect of processing variables on the physico-chemical characteristics and aroma of borş , a traditional beverage derived from wheat bran. Food Chemistry 2018, 265, 242-252, 10.1016/j.foodchem.2018.05.095.
- Silvia-Simona Grosu-Tudor; Iulia-Roxana Stefan; Mihaela-Marilena Stancu; Calina-Petruta Cornea; Luc De Vuyst; Medana Zamfir; Microbial and nutritional characteristics of fermented wheat bran in traditional Romanian borş production. ROMANIAN BIOTECHNOLOGICAL LETTERS 2019, 24, 440-447, 10.25083/rbl/24.3/440.447.
- Sirma Yegin; Ali Üren; Biogenic amine content of boza: A traditional cereal-based, fermented Turkish beverage. Food Chemistry 2008, 111, 983-987, 10.1016/j.foodchem.2008.05.020.
- Gopal Kedia; Ruohang Wang; Hemant Patel; Severino S. Pandiella; Use of mixed cultures for the fermentation of cereal-based substrates with potential probiotic properties. Process Biochemistry 2007, 42, 65-70, 10.1016/j.procbio.2006.07.011.
- Bamforth, C.W. Barley and Malt Starch in Brewing: A General Review; Technical quarterly-Master Brewers Association of the Americas: Madison, WI, USA, 2003; Volume 40, pp. 89–97.
- Jane Misihairabgwi; Natascha Cheikhyoussef; Traditional fermented foods and beverages of Namibia. Journal of Ethnic Foods 2017, 4, 145-153, 10.1016/j.jef.2017.08.001.
- Ramakrishnan, S.R.; Ravichandran, K.; Antony, U.M. Whole Grains: Processing, Product Development, and Nutritional Aspects; Mir, S.A., Manickavasagan, A., Shah, M.A., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 103–127
- Yeonhee Lee; Characterization ofWeissella kimchiiPL9023 as a potential probiotic for women. FEMS Microbiology Letters 2005, 250, 157-162, 10.1016/j.femsle.2005.07.009.
- G. Vieira-Dalodé; Lene Jespersen; J. Hounhouigan; P.L. Möller; C.M. Nago; M. Jakobsen; Lactic acid bacteria and yeasts associated withgowéproduction from sorghum in Bénin. Journal of Applied Microbiology 2007, 103, 342-349, 10.1111/j.1365-2672.2006.03252.x.
- Vivekananda Mandal; Sukanta Kumar Sen; Vivekananda Mandal; Isolation and Characterization of Pediocin NV 5 Producing Pediococcus acidilactici LAB 5 from Vacuum-Packed Fermented Meat Product. Indian Journal of Microbiology 2011, 51, 22-29, 10.1007/s12088-011-0070-0.
- Laurent Adinsi; Noël H. Akissoe; Générose Dalodé-Vieira; Victor B. Anihouvi; Genevieve Fliedel; Christian Mestres; Joseph D. Hounhouigan; Sensory evaluation and consumer acceptability of a beverage made from malted and fermented cereal: case of gowe from Benin. Food Science & Nutrition 2014, 3, 1-9, 10.1002/fsn3.166.
- Laurent Adinsi; Christian Mestres; Noël Akissoé; Générose Vieira-Dalodé; Victor Anihouvi; Noël Durand; D. Joseph Hounhouigan; Comprehensive quality and potential hazards of gowe, a malted and fermented cereal beverage from West Africa. A diagnostic for a future re-engineering. Food Control 2017, 82, 18-25, 10.1016/j.foodcont.2017.06.019.
- Agarry, O.O.; Nkama, I.; Akoma, O.; Production of Kunun-zaki (A Nigerian fermented cereal beverage) using starter culture. Int. Res. J. Microbiol. 2010, 1, 18–25. .
- Nkama, I.; Agarry, O.O.; Akoma, O.; Sensory and nutritional quality characteristics of powdered‘ Kunun-zaki’: A Nigerian fermented cereal beverage. Afr. J. Food Sci. 2010, 4, 364–370.
- Wartu, J.R.; Whong, C.M.Z.; Abdullahi, I.O.; Ameh, J.B.; Musa, B.J.; TRENDS IN TOTAL AFLATOXINS AND NUTRITIONAL IMPACT OFTRITICUM SPP DURING FERMENTATION OF KUNUN-ZAKI; A NIGERIA SORGHUM BICOLOR BASED NON-ALCOHOLIC BEVERAGE. Int. J. Life Sci. Pharma Res. 2015, 5, 26–35.
- Adeniji Paulina Olufunke; Keshinro Oluremi O; Assessment of Nutritional and Sensory Quality of Kunun Zaki - A Homemade Traditional Nigerian Beverage. Journal of Nutrition & Food Sciences 2014, 5, 3-5, 10.4172/2155-9600.1000358.
- Loreta Basinskiene; Grazina Juodeikiene; Daiva Vidmantiene; Maija Tenkanen; Tomas Makaravicius; Elena Bartkiene; Non-Alcoholic Beverages from Fermented Cereals with Increased Oligosaccharide Content. Food Technology and Biotechnology 2016, 54, 36-44, 10.17113/ftb.54.01.16.4106.
- Halina Gambuś; Barbara Mickowska; Henryk Bartoń; Grażyna Augustyn; Gabriela Zięć; Dorota Litwinek; Katarzyna Szary-Sworst; Wiktor Berski; Health benefits of kvass manufactured from rye wholemeal bread. Journal of Microbiology, Biotechnology and Food Sciences 2015, 4, 34-39, 10.15414/jmbfs.2015.4.special3.34-39.
- J.M Bvochora; J.D Reed; J.S Read; Remigio Zvauya; Effect of fermentation processes on proanthocyanidins in sorghum during preparation of Mahewu, a non-alcoholic beverage. Process Biochemistry 1999, 35, 21-25, 10.1016/s0032-9592(99)00027-8.
- Idowu, O.O.; Fadahunsi, I.F.; Onabiyi, O.A.; A Production and Nutritional Evaluation of Mahewu: A Non-Alcoholic Fermented Beaverage of South Africa. Int. J. Res. Pharm. Biosci 2016, 3, 27.
- Elsa Maria Salvador; Cme McCrindle; Elna M. Buys; Vanessa Steenkamp; Standardization of cassava Mahewu fermentation and assessment of the effects of iron sources used for fortification. African Journal of Food, Agriculture, Nutrition and Development 2016, 16, 10898-10912, 10.18697/ajfand.74.15305.
- Ilesanmi Festus Fadahunsi; Opeyemi Olayinka Soremekun; Production, Nutritional and Microbiological Evaluation of Mahewu a South African Traditional Fermented Porridge. Journal of Advances in Biology & Biotechnology 2017, 14, 1-10, 10.9734/jabb/2017/33175.
- Roland Kibwega Foma; Jacqueline Destain; Paul Kapay Mobinzo; Kalenga Kayisu; Phillipe Thonart; Study of physicochemical parameters and spontaneous fermentation during traditional production of munkoyo, an indigenous beverage produced in Democratic Republic of Congo. Food Control 2012, 25, 334-341, 10.1016/j.foodcont.2011.10.049.
- R.M. Zulu; V.M. Dillon; J.D. Owens; Munkoyo beverage, a traditional Zambian fermented maize gruel using Rhynchosia root as amylase source. International Journal of Food Microbiology 1997, 34, 249-258, 10.1016/s0168-1605(96)01195-6.
- Justin Chileshe; Joost Van Den Heuvel; Ray Handema; Bas J. Zwaan; Elise F Talsma; Sijmen E. Schoustra; Nutritional Composition and Microbial Communities of Two Non-alcoholic Traditional Fermented Beverages from Zambia: A Study of Mabisi and Munkoyo. Nutrients 2020, 12, 1628, 10.3390/nu12061628.
- Srikaeo, K. Biotechnological Tools in the Production of Functional Cereal-Based Beverages; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128166789
- J. M. Misihairabgwi; A. Ishola; I. Quaye; Michael Sulyok; R. Krska; Diversity and fate of fungal metabolites during the preparation of oshikundu, a Namibian traditional fermented beverage. World Mycotoxin Journal 2018, 11, 471-481, 10.3920/wmj2018.2352.
- W. Embashu; Komeine K.M. Nantanga; Pearl millet grain: A mini-review of the milling, fermentation and brewing of ontaku, a non-alcoholic traditional beverage in Namibia. Transactions of the Royal Society of South Africa 2019, 74, 276-282, 10.1080/0035919x.2019.1650310.
- Nabil Ben Omar; Frédéric Ampe; Microbial Community Dynamics during Production of the Mexican Fermented Maize Dough Pozol. Applied and Environmental Microbiology 2000, 66, 3664-3673, 10.1128/aem.66.9.3664-3673.2000.
- Todorov, S.D.; Holzapfel, W.H. Traditional Cereal Fermented Foods as Sources of Functional Microorganisms; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9781782420248.
- Filiz Altay; Funda Karbancıoğlu-Güler; Ceren Daskaya-Dikmen; Dilek Heperkan; A review on traditional Turkish fermented non-alcoholic beverages: Microbiota, fermentation process and quality characteristics. International Journal of Food Microbiology 2013, 167, 44-56, 10.1016/j.ijfoodmicro.2013.06.016.
- Fatma Çoşkun; A Traditional Turkish Fermented Non-Alcoholic Beverage, “Shalgam”. Beverages 2017, 3, 49, 10.3390/beverages3040049.
- T Gadaga; A review of traditional fermented foods and beverages of Zimbabwe. International Journal of Food Microbiology 1999, 53, 1-11, 10.1016/s0168-1605(99)00154-3.
- Yasuyuki Oi; Naofumi Kitabatake; Chemical Composition of an East African Traditional Beverage,Togwa. Journal of Agricultural and Food Chemistry 2003, 51, 7024-7028, 10.1021/jf0203343.
- Deborah M. Waters; Alexander Mauch; Aidan Coffey; Elke K. Arendt; Emanuele Zannini; Lactic Acid Bacteria as a Cell Factory for the Delivery of Functional Biomolecules and Ingredients in Cereal-Based Beverages: A Review. Critical Reviews in Food Science and Nutrition 2014, 55, 503-520, 10.1080/10408398.2012.660251.
- Ajiro, M.; Araki, M.; Ishikawa, M.; Kobayashi, K.; Ashida, I.; Miyaoka, Y. Analysis of Functional Relationships between Rice Particles and Oral Perception Using Amazake: A Traditional Japanese Beverage of Malted Rice. Food Nutr. Sci. 2017, 08, 901–911.
- Kawakami, S.; Ito, R.; Maruki-Uchida, H.; Kamei, A.; Yasuoka, A.; Toyoda, T.; Ishijima, T.; Nishimura, E.; Morita, M.; Sai, M.; et al. Intake of a mixture of sake cake and rice malt increases mucin levels and changes in intestinal microbiota in mice. Nutrients 2020, 12, 449
- Petrova, P.; Petrov, K. Traditional cereal Beverage Boza: Fermentation technology, microbial content and healthy effects. In Fermented Foods Part II Technological Interventions; Ray, R.C., Montet, D., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 284–305.
- M. Ajiro; M. Araki; M. Ishikawa; K. Kobayashi; I. Ashida; Y. Miyaoka; Analysis of Functional Relationships between Rice Particles and Oral Perception Using Amazake: A Traditional Japanese Beverage of Malted Rice. Food and Nutrition Sciences 2017, 8, 901-911, 10.4236/fns.2017.810065.
- Shinpei Kawakami; Ryouichi Ito; Hiroko Maruki-Uchida; Asuka Kamei; Akihito Yasuoka; Tsudoi Toyoda; Tomoko Ishijima; Eisaku Nishimura; Minoru Morita; Masahiko Sai; et al.Keiko AbeShinji Okada Intake of a Mixture of Sake Cake and Rice Malt Increases Mucin Levels and Changes in Intestinal Microbiota in Mice. Nutrients 2020, 12, 449, 10.3390/nu12020449.
- Yoshifumi Oguro; Toshikazu Nishiwaki; Ryota Shinada; Kazuya Kobayashi; Atsushi Kurahashi; Metabolite profile of koji amazake and its lactic acid fermentation product by Lactobacillus sakei UONUMA. Journal of Bioscience and Bioengineering 2017, 124, 178-183, 10.1016/j.jbiosc.2017.03.011.
- Sahu, L.; Panda, S.K. Innovative Technologies and Implications in Fermented Food and Beverage Industries: An Overview. In Innovations in Technologies for Fermented Food and Beverage Industries; Springer: Berlin, Germany, 2018; pp. 1–23.
- Arzu Akpinar-Bayizit; Lutfiye Yilmaz-Ersan; Tulay Ozcan; Determination of Boza's Organic Acid Composition as it is Affected by Raw Material and Fermentation. International Journal of Food Properties 2010, 13, 648-656, 10.1080/10942911003604194.
- Velitchka Gotcheva; Zlatka Roshkova; Colin Webb; S.S Pandiella; Angel Angelov; Monitoring the fermentation of the traditional Bulgarian �beverage boza. International Journal of Food Science & Technology 2001, 36, 129-134, 10.1046/j.1365-2621.2001.00429.x.
- I.M. Mukisa; D.G. Nsiimire; Yusuf B. Byaruhanga; C.M.B.K. Muyanja; T. Langsrud; J.A. Narvhus; OBUSHERA: DESCRIPTIVE SENSORY PROFILING AND CONSUMER ACCEPTABILITY. Journal of Sensory Studies 2010, 25, 190–214, 10.1111/j.1745-459x.2009.00272.x.
- Jean-Pierre, G.; Serge, T.; Dolores, R.; Judith, E.; Dora, C.; Carmen, W.; Pozol, a popular Mexican traditional beverage made from a fermented alkaline cooked maize dough. Food Besed Approch. Health Nutr. 2003, 11, 23–28.
- Janyl Iskakova; Jamila Smanalieva; Frank-Juergen Methner; Investigation of changes in rheological properties during processing of fermented cereal beverages. Journal of Food Science and Technology 2019, 56, 3980-3987, 10.1007/s13197-019-03865-9.
- R. Nyanzi; P.J. Jooste; Cereal-Based Functional Foods. Probiotics 2012, null, 161–197, 10.5772/50120.
- Smith G. Nkhata; Emmanuel Ayua; Elijah H. Kamau; Jean-Bosco Shingiro; Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Science & Nutrition 2018, 6, 2446-2458, 10.1002/fsn3.846.
- Gillooly, M.; Bothwell, T.H.; Charlton, R.W.; Torrance, J.D.; Bezwoda, W.R.; MacPhail, A.P.; Derman, D.P.; Novelli, L.; Morrall, P.; Mayet, F. Factors affecting the absorption of iron from cereals. Br. J. Nutr. 1984, 51, 37–46.
- Khetarpaul, N.; Chauhan, B.M. Effect of fermentation by pure cultures of yeasts and lactobacilli on the available carbohydrate content of pearl millet. Food Chem. 1990, 36, 287–293.
- Nout, M.J.R.; Motarjemi, Y. Assessment of fermentation as a household technology for improving food safety: A joint FAO/WHO workshop. Food Control 1997, 8, 221–226
- Naofumi Kitabatake; Dorothy M. Gimbi; Yasuyuki Oi; Traditional non-alcoholic beverage,Togwa, in East Africa, produced from maize flour and germinated finger millet. International Journal of Food Sciences and Nutrition 2003, 54, 447-455, 10.1080/09637480120092053.
- Papageorgiou, M.; Skendi, A. Introduction to Cereal Processing and By-Products; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780081022146
- Youna Hemery; Marc Chaurand; Ulla R. M. Holopainen; Anna-Maija Lampi; Pekka Lehtinen; Vieno Piironen; Abdelkrim Sadoudi; Xavier Rouau; Potential of dry fractionation of wheat bran for the development of food ingredients, part I: Influence of ultra-fine grinding. Journal of Cereal Science 2011, 53, 1-8, 10.1016/j.jcs.2010.09.005.
- Kunze, W. Technology Brewing and Malting; VLB Berlin: Berlin, Germany, 2004; Volume 949.
- Laurent Adinsi; Générose Vieira-Dalode; Noël Akissoé; Victor Anihouvi; Christian Mestres; Annali Jacobs; Nomusa Dlamini; Dominique Pallet; D. Joseph Hounhouigan; Processing and quality attributes of gowe: a malted and fermented cereal-based beverage from Benin. Food Chain 2014, 4, 171-183, 10.3362/2046-1887.2014.016.
- M.A Igyor; A.C Ogbonna; G.H. Palmer; Effect of malting temperature and mashing methods on sorghum wort composition and beer flavour. Process Biochemistry 2001, 36, 1039-1044, 10.1016/s0032-9592(00)00267-3.
- Foma, R.K.; Destain, J.; Mobinzo, P.K.; Kayisu, K.; Thonart, P. Study of physicochemical parameters and spontaneous fermentation during traditional production of munkoyo, an indigenous beverage produced in Democratic Republic of Congo. Food Control 2012, 25, 334–341.
- Zulu, R.M.; Dillon, V.M.; Owens, J.D. Munkoyo beverage, a traditional Zambian fermented maize gruel using Rhynchosia root as amylase source. Int. J. Food Microbiol. 1997, 34, 249–258.
- Elena Dlusskaya; André Jänsch; Clarissa Schwab; Michael G. Gänzle; Microbial and chemical analysis of a kvass fermentation. European Food Research and Technology 2007, 227, 261-266, 10.1007/s00217-007-0719-4.
- Krebs, G.; Becker, T.; Gastl, M. Characterization of polymeric substance classes in cereal-based beverages using asymmetrical flow field-flow fractionation with a multi-detection system. Anal. Bioanal. Chem. 2017, 409, 5723–5734.
- Krebs, G.; Müller, M.; Becker, T.; Gastl, M. Characterization of the macromolecular and sensory profile of non-alcoholic beers produced with various methods. Food Res. Int. 2019, 116, 508–517.
- Sorelle Nsogning Dongmo; Susanne Procopio; Bertram Sacher; Thomas Becker; Flavor of lactic acid fermented malt based beverages: Current status and perspectives. Trends in Food Science & Technology 2016, 54, 37-51, 10.1016/j.tifs.2016.05.017.
- Carvalho, D.O.; Gonçalves, L.M.; Guido, L.F. Overall Antioxidant Properties of Malt and How They Are Influenced by the Individual Constituents of Barley and the Malting Process. Compr. Rev. Food Sci. Food Saf. 2016, 15, 927–943.
- Wannenmacher, J.; Gastl, M.; Becker, T. Phenolic Substances in Beer: Structural Diversity, Reactive Potential and Relevance for Brewing Process and Beer Quality. Compr. Rev. Food Sci. Food Saf. 2018, 17, 953–988.
- Mahesh Gupta; Nissreen Abu‐Ghannam; Eimear Gallaghar; Barley for Brewing: Characteristic Changes during Malting, Brewing and Applications of its By-Products. Comprehensive Reviews in Food Science and Food Safety 2010, 9, 318-328, 10.1111/j.1541-4337.2010.00112.x.
- Krebs, G.; Becker, T.; Gastl, M. Influence of malt modification and the corresponding macromolecular profile on palate fullness in cereal-based beverages. Eur. Food Res. Technol. 2020, 246, 1219–1229.
- Liptáková, D.; Matejčeková, Z.; Valík, L. Lactic Acid Bacteria and Fermentation of Cereals and Pseudocereals. Ferment. Process. 2017, 223–254.
- Fărcaş, A.; Tofană, M.; Socaci, S.; Mudura, E.; Scrob, S.; Salanţă, L.; Mureşan, V.; Brewers’ spent grain—A new potential ingredient for functional foods. . Agroaliment. Process. Technol. 2014, 20, 137–141.
- Nachel, M. Homebrewing for Dummies, 2nd ed.; Wiley Publishing, Inc.: Hoboken, NJ, USA, 2008; ISBN 9780470230626.
- Shannon Rezac; Car Reen Kok; Melanie Heermann; Robert Hutkins; Fermented Foods as a Dietary Source of Live Organisms. Frontiers in Cellular and Infection Microbiology 2018, 9, 1785, 10.3389/fmicb.2018.01785.
- Motarjemi, Y.; Nout, M.J.R.; Food fermentation: A safety and nutritional assessment. Bull. World Health Organ. 1996, 74, 553–559.
- Oylum Erkus; Victor Cl De Jager; Maciej Spus; Ingrid J Van Alen-Boerrigter; Irma Mh Van Rijswijck; Lucie Hazelwood; Patrick Wm Janssen; Sacha A. F. T. Van Hijum; Michiel Kleerebezem; Eddy J. Smid; et al. Multifactorial diversity sustains microbial community stability. The ISME Journal 2013, 7, 2126-2136, 10.1038/ismej.2013.108.
- Stacey Butler; James P. O’Dwyer; Stability criteria for complex microbial communities.. Nature Communications 2018, 9, 2970, 10.1038/s41467-018-05308-z.
- Taylor, J.R.N. Fermentation: Foods and Nonalcoholic Beverages, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 3–4, ISBN 9780123947864.
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243.
- Kohajdov, Z.; Karovi, J.; Fermentation of cereals for specific purpose. J. Food Nutr. Res. 2007, 46, 51-57.
- Fernandes, C.G.; Sonawane, S.K.; Arya, S.S.; Cereal based functional beverages: A review.. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 914–919.
- Aka, S.; Konan, G.; Fokou, G.; Dje Koffi, M.; Bonfoh, B. Review on African traditional cereal beverages. Am. J. Res. Commun. 2014, 2, 103–153.
- Mugula, J.K.; Nnko, S.A.M.; Narvhus, J.A.; Sørhaug, T. Microbiological and fermentation characteristics of togwa, a Tanzanian fermented food. Int. J. Food Microbiol. 2003, 80, 187–199.
- Macori, G.; Cotter, P.D. Novel insights into the microbiology of fermented dairy foods. Curr. Opin. Biotechnol. 2018, 49, 172–178.
- Smid, E.J.; Hugenholtz, J. Functional Genomics for Food Fermentation Processes. Annu. Rev. Food Sci. Technol. 2010, 1, 497–519
- Theron, M.; Lues, J.R. Organic Acids and Food Preservation; CRC Press: Boca Raton, FL, USA, 2010
- Thomas Bintsis; Foodborne pathogens.. AIMS Microbiology 2017, 3, 529-563, 10.3934/microbiol.2017.3.529.
- Stellah Byakika; Ivan Muzira Mukisa; Yusuf Byenkya Byaruhanga; Denis Male; Charles Muyanja; Influence of food safety knowledge, attitudes and practices of processors on microbiological quality of commercially produced traditional fermented cereal beverages, a case of Obushera in Kampala. Food Control 2019, 100, 212-219, 10.1016/j.foodcont.2019.01.024.
- Jyoti Prakash Tamang; Paul D. Cotter; Akihito Endo; Nam Soo Han; Remco Kort; Shao Quan Liu; Baltasar Mayo; Nieke Westerik; Robert Hutkins; Fermented foods in a global age: East meets West. Comprehensive Reviews in Food Science and Food Safety 2020, 19, 184-217, 10.1111/1541-4337.12520.
- D.I. Gernah; C.C. Ariahu; E.K. Ingbian; Effects of Malting and Lactic Fermentation on Some Chemical and Functional Properties of Maize (Zea mays). American Journal of Food Technology 2011, 6, 404-412, 10.3923/ajft.2011.404.412.
- Sharaf, O.M.; El-Shafei, K.; Ibrahim, G.A.; El-Sayed, H.S.; Kassem, J.M.; Assem, F.M.; Tawfek, N.F.; Effat, B.A.; Abd El-Khalek, A.B.; Dabiza, N. Preparation, properties and evaluation of folate and riboflavin enriched six functional cereal-fermented milk beverages using encapsulated Lactobacillus plantarum or Streptococcus thermophiles. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1724–1735.
- Chavan, M.; Gat, Y.; Harmalkar, M.; Waghmare, R. Development of non-dairy fermented probiotic drink based on germinated and ungerminated cereals and legume. LWT Food Sci. Technol. 2018, 91, 339–344.
- Achi, O.K.; The potential for upgrading traditional fermented foods through biotechnology. Afr. J. Biotechnol. 2005, 4, 375–380.
- Pasquale Russo; Mattia Pia Arena; Daniela Fiocco; Vittorio Capozzi; Djamel Drider; Giuseppe Spano; Lactobacillus plantarum with broad antifungal activity: A promising approach to increase safety and shelf-life of cereal-based products. International Journal of Food Microbiology 2017, 247, 48-54, 10.1016/j.ijfoodmicro.2016.04.027.
- Angel Angelov; Teodora Yaneva-Marinova; Velitchka Gotcheva; Oats as a matrix of choice for developing fermented functional beverages. Journal of Food Science and Technology 2018, 55, 2351-2360, 10.1007/s13197-018-3186-y.
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food fermentations: Microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012, 154, 87–97.
- Ogunremi, O.R.; Banwo, K.; Sanni, A.I. Starter-culture to improve the quality of cereal-based fermented foods: Trends in selection and application. Curr. Opin. Food Sci. 2017, 13, 38–43.
- Müge Gök Charyyev; Banu Özden Tuncer; Didem Akpınar Kankaya; Yasin Tuncer; Bacteriocinogenic properties and safety evaluation of Enterococcus faecium YT52 isolated from boza, a traditional cereal based fermented beverage. Journal für Verbraucherschutz und Lebensmittelsicherheit 2019, 14, 41-53, 10.1007/s00003-019-01213-9.
- Sultan Arslan-Tontul; Mustafa Erbaş; Co-Culture Probiotic Fermentation of Protein-Enriched Cereal Medium (Boza). Journal of the American College of Nutrition 2019, 39, 72-81, 10.1080/07315724.2019.1612796.
- Paulina Markowiak; K. Slizewska; Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021, 10.3390/nu9091021.
- Kaur, P.; Ghoshal, G.; Banerjee, U.C. Traditional Bio-Preservation in Beverages: Fermented Beverages; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128166857.
- Simango, C. Lactic acid fermentation of sour porridge and mahewu, a non-alcoholic fermented cereal beverage. J. Appl. Sci. S. Afr. 2002, 08, 89–98.
- Alba Yépez; Pasquale Russo; Giuseppe Spano; Iuliia Khomenko; Franco Biasioli; Vittorio Capozzi; Rosa Aznar; Franco Biasoli; In situ riboflavin fortification of different kefir-like cereal-based beverages using selected Andean LAB strains. Food Microbiology 2019, 77, 61-68, 10.1016/j.fm.2018.08.008.
- Adekemi Titilayo Adesulu-Dahunsi; Samuel Olatunde Dahunsi; Adeniyi Olayanju; Synergistic microbial interactions between lactic acid bacteria and yeasts during production of Nigerian indigenous fermented foods and beverages. Food Control 2020, 110, 106963, 10.1016/j.foodcont.2019.106963.
- M. Salari; S.H. Razavi; Seyed Mohammad Taghi Gharibzahedi; Characterising the synbiotic beverages based on barley and malt flours fermented by Lactobacillus delbrueckii and paracasei strains. Quality Assurance and Safety of Crops & Foods 2015, 7, 355-361, 10.3920/qas2013.0390.
- Remco Kort; Wilbert Sybesma; Probiotics for every body. Trends in Biotechnology 2012, 30, 613-615, 10.1016/j.tibtech.2012.09.002.
- M. C. Setta; A. Matemu; E. R. Mbega; Potential of probiotics from fermented cereal-based beverages in improving health of poor people in Africa. Journal of Food Science and Technology 2020, null, 1-12, 10.1007/s13197-020-04432-3.
- Abul Kalam Azad; Manobendro Sarker; Dan Wan; Immunomodulatory Effects of Probiotics on Cytokine Profiles. BioMed Research International 2018, 2018, 1-10, 10.1155/2018/8063647.
- Nelson Mota De Carvalho; E.M. Costa; Sara Silva; Lígia Pimentel; Tito Fernandes; Manuela Pintado; Fermented Foods and Beverages in Human Diet and Their Influence on Gut Microbiota and Health. Fermentation 2018, 4, 90, 10.3390/fermentation4040090.
- Ome K. Achi; Naomi U. Asamudo; Cereal-Based Fermented Foods of Africa as Functional Foods. Reference Series in Phytochemistry 2019, null, 1527-1558, 10.1007/978-3-319-78030-6_31.
- Vittorio Capozzi; Pasquale Russo; M.Teresa Dueñas-Chasco; Paloma López; Giuseppe Spano; Lactic acid bacteria producing B-group vitamins: a great potential for functional cereals products. Applied Microbiology and Biotechnology 2012, 96, 1383-1394, 10.1007/s00253-012-4440-2.
- Tannock, G.W.; Identification of Lactobacilli and Bifidobacteria. Current Issues in Molecular Biology 1999, 1, 53–64, 10.21775/cimb.001.053.
- Jiwan S. Sidhu; Yearul Kabir; Fatma G. Huffman; Functional Foods from Cereal Grains. International Journal of Food Properties 2007, 10, 231-244, 10.1080/10942910601045289.
- Elena Mudura; Teodora Coldea; Carmen Socaciu; Floricuţa Ranga; Carmen Pop; Ancuţa Rotar; Antonella Pasqualone; Brown beer vinegar: A potentially functional product based on its phenolic profile and antioxidant activity. Journal of the Serbian Chemical Society 2018, 83, 19-30, 10.2298/jsc170803107m.
- Elena Mudura; Teodora Coldea; Carmen Socaciu; Floricuţa Ranga; Carmen Pop; Ancuţa Rotar; Antonella Pasqualone; Brown beer vinegar: A potentially functional product based on its phenolic profile and antioxidant activity. Journal of the Serbian Chemical Society 2018, 83, 19-30, 10.2298/jsc170803107m.
- Jean-Pierre, G.; Serge, T.; Dolores, R.; Judith, E.; Dora, C.; Carmen, W.; Pozol, a popular Mexican traditional beverage made from a fermented alkaline cooked maize dough. Food Besed Approch. Health Nutr. 2003, 11, 23–28.
