Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Hui Liu.
Coriander or Coriandrum sativum (C. sativum), a member of the Apiaceae family, is a popular herb with versatile applications. The seeds and leaves are widely used for culinary and seasoning. The seeds and fruits are often used for cooking meat. As honey plant, coriander is highly attractive to honeybee workers. Additionally, coriander has been used in many traditional medicines, and its medicinal values has been widely recognized. Coriander extracts have a wide range of biological benefits including neuroprotective, anxiolytic, hypnotic, antioxidant, anti-inflammatory, and so on.
- coriander plant
- emotion
- brain electrophysiology
- salivary secretion
1. Introduction
People in modern society are under greater life and work pressures, which will affect their physical and mental health and even increase the risk of depression [1,2][1][2]. Depression is a persistent and serious mental illness that affects over 120 million people worldwide [2]. According to the World Health Organization statistics, depression will be the leading cause of death worldwide by 2030 [3,4][3][4]. According to the reports, the direct or indirect treatment costs of depression are more than $30 billion each year in the United States, which will lead to a significant social burden [5]. Moreover, most of the treated depression patients often have residual symptoms that persist, leading to impaired social function of the patients. Therefore, the prevention of depression is particularly important [6].
In stressful modern life, the relaxing effect of natural stimuli is considered beneficial compared with other stimuli [1]. According to stress recovery theory, exposure to unthreatening nature can elicit positive emotions, restrict negative thoughts, and reduces stress [7]. Many people are thus attracted to the physiological and psychological relaxing effect of exposure to plants. Field experiments in urban parks and forest bathing have proved the psychological and physical relaxing effects of contact with plants [8,9][8][9]. Forest bathing also increased natural killer cell function and improved immune function [10]. This effect was sustained for approximately 1 month. Indeed, urban residents were socially isolated during the outbreak of the Covid-19 virus outbreak, and the value of residential gardens as therapeutic landscapes was brought to the fore [11]. These results suggest that contact with plants is a type of prophylaxis.
The color and volatile organic compounds (VOCs) of plants may play an important role in regulating emotions. People mainly achieve the color perception through light, and different colors of plants reflect different wavelengths of light [12]. Intrinsically photosensitive retinal ganglion cells (ipRGCs) sense light and project it to the paraventricular nucleus of the hypothalamus. This area regulates the secretion of stress hormones by stimulating the adrenal gland [13]. Stress hormone levels can reflect stressful, emotional conditions [14]. Previous research has shown that the green color of plants can effectively restore workers’ brainwave and the mental state, reduce shoulders and back pain, and relieve their work pressure [15]. The volatile organic compounds of plants are beneficial to people’s physiological and psychological health. They can relieve anxiety and depression and maintain the memory of patients with Alzheimer’s disease or other memory disorders [16].
Coriander or Coriandrum sativum (C. sativum), a member of the Apiaceae family, is a popular herb with versatile applications. The seeds and leaves are widely used for culinary and seasoning. The seeds and fruits are often used for cooking meat [17]. As honey plant, coriander is highly attractive to honeybee workers [18]. Additionally, coriander has been used in many traditional medicines, and its medicinal values has been widely recognized [19,20][19][20]. Coriander extracts have a wide range of biological benefits including neuroprotective, anxiolytic, hypnotic, antioxidant, anti-inflammatory, and so on [21,22][21][22]. However, the current research about coriander medicinal values have been mainly focusing on its extracts while lacking on living coriander plants. Our previous study found that coriander plants had a potential role in regulating negative emotions [12]. Therefore, this study aims to investigate the effects of coriander plants on human emotions and physiological activities and the correlation between emotional fluctuations and salivary secretion.
2. Main VOCs of Coriander Plants
Coriander plants VOCs was analyzed by GC-MS. Representative gas chromatography–mass spectrometry (GC–MS) total ion chromatograms (Figure S1 could be found https://www.mdpi.com/2079-7737/10/12/1283#supplementary). From the main of 22 separated peaks, alcohols, terpenoids, and esters were the predominant class of compounds, with 2-ethyl-1-hexanol (15.60%) being the major component (Table 21). 2-ethyl-1-hexanol was often found in the natural VOCs of many plants. Other important substances were: d-limonene (9.58%), eucalyptol (8.97%), benzyl alcohol (6.16%), isophorone (6.06%), dimethyl glutarate (5.03%), α-terpineol (4.45%), styrene (3.97%), methyl methacrylate (3.20%), α-pinene (3.17%). The concentration of VOCs per cm3 of coriander plants in the test room was about 0.82 ng. The smell of d-limonene was described as a light lemon smell, α-pinene having a moderate resin scent.
Table 21. Main volatile components of coriander plants.
| Compounds | Chemical Formula | RT (min) | RI | RC (%) | CAS |
|---|---|---|---|---|---|
| Longifolene | C15H24 | 36.46 | 1417.58 | 2.20 | 475-20-7 |
| 1,3,5-Benzetriol, 3TMS derivative | C15H30O3Si3 | 32.49 | 1265.55 | 1.66 | 10586-12-6 |
| 2-Ethylhexyl acrylate | C11H20O2 | 30.82 | 1146.97 | 1.59 | 103-11-7 |
| α-Terpineol | C10H18O | 29.88 | 1136.51 | 4.45 | 98-55-5 |
| Dimethyl glutarate | C7H12O4 | 27.86 | 1114.01 | 5.03 | 1119-40-0 |
| Lsophorone | C9H14O | 27.59 | 1110.99 | 6.06 | 78-59-1 |
| 1,2,3,5-Tetramethylbenzene | C10H14 | 27.37 | 1108.63 | 2.82 | 527-53-7 |
| 3-Hydroxymandelic acid, 3TMS derivative | C17H32O4Si3 | 27.22 | 1106.86 | 2.69 | 68595-69-7 |
| Linalool | C10H18O | 26.66 | 1100.66 | 0.60 | 78-70-6 |
| Acetophenone | C8H8O | 25.58 | 987.55 | 1.95 | 98-86-2 |
| γ-Terpinene | C10H16 | 25.27 | 983.73 | 1.10 | 99-85-4 |
| Benzyl alcohol | C7H8O | 24.36 | 972.51 | 6.16 | 100-51-6 |
| Eucalyptol | C10H18O | 24.30 | 971.76 | 8.87 | 470-82-6 |
| D-Limonene | C10H16 | 24.16 | 870.37 | 9.58 | 5989-27-5 |
| 2-Ethyl-1-hexanol | C8H18O | 24.07 | 968.90 | 15.60 | 104-76-7 |
| α-Pinene | C10H16 | 20.17 | 930.96 | 3.17 | 80-56-8 |
| Styrene | C8H8 | 18.11 | 805.88 | 3.92 | 100-42-5 |
| Furfural | C5H4O2 | 15.14 | 639.22 | 0.75 | 98-01-1 |
| Methyl methacrylate | C5H8O2 | 9.18 | 613.16 | 3.20 | 80-62-6 |
| 1-Butanol | C4H10O | 7.59 | 692.01 | 1.45 | 71-36-3 |
| 1,2-Ethanediol, diformate | C4H6O4 | 6.35 | 598.03 | 6.18 | 629-15-2 |
| Ethyl Acetate | C4H8O2 | 6.61 | 618.02 | 2.54 | 141-78-6 |
| Other volatile components | - | - | t | - |
RT = retention times; RI = retention indices; RC = relative concentration; t—trace (<0.06%).
3. Effects of Coriander Plants on Subjective Emotion
More positive psychological relaxation occurred for the stimulus involving coriander plants (Figure 21). Among the subcategories, A-H was lower when performing the task in the coriander group and there was a statistically significant difference (p < 0.05, 95% Cl = 0.01; 1.18, Cohen’s d = 1.62, r = 0.63). Regarding other negative emotions and TMD scores, the coriander group tended to be lower than the control group, but there was no statistical difference.
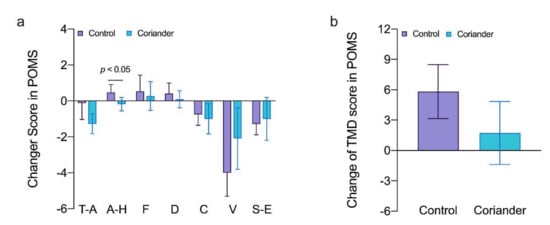

Figure 21. (a) Subscale scores for the profile of mood states (POMS) scale in the coriander and control groups (means ± SD). T-A, tension-anxiety; A-H, anger-hostility; F, fatigue; D, dejection; C, confusion; V, vigor; S-E, self-esteem; (b) Comparison of the total mood disturbance (TMD) in the profile of mood state (POMS) questionnaire in the two groups. Data are presented as variation ± standard error (n = 13). Independent t-tests were used to identify the significant differences between the two groups.
4. Effects of Coriander Plants on EEG
The EEG power of theta and alpha was calculated for the control and coriander groups before and after the experiment. Figure 32 and Table S1 show the power of theta brainwaves in two groups, and Figure 43 presents the topographic map. The topographic map showed an obvious change in theta power in the coriander group, particularly in the frontal, frontal central, central, and central parietal areas. The alpha wave power change also showed the same trend as theta, but the difference was not significant (Figure S2).
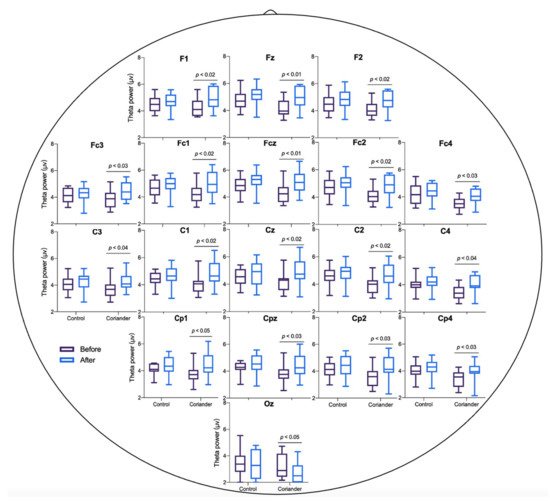
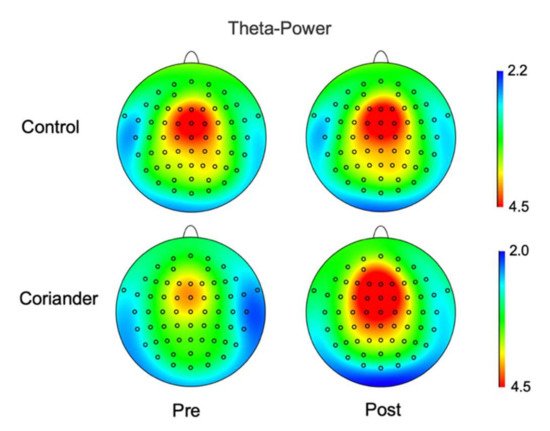

Figure 32. Theta wave power before and after homework in the group of coriander and control. The mean power in theta waves during the sit still for 5 min. Data presented as variation ± standard error (n = 10). Paired t-tests were used to identify the significant differences before and after in the same groups.

Figure 43. Theta wave power maps before and after homework in the coriander and control groups. The mean power in theta waves during the sit still for 5 min was analyzed and mapped it on the brain. Data presented as a variation (n = 10).
5. Effects of Coriander on Salivary Alpha-Amylase and Cortisol
Salivary alpha-amylase and cortisol indicated the physiological changes related to emotion. Figure 54 shows the variation in salivary-alpha amylase and cortisol concentrations after the home work in the two groups. Salivary alpha-amylase and cortisol concentrations showed a reducing trend in the coriander groups, especially the salivary alpha-amylase level (p < 0.01, 95% Cl = 132.33; 372.82, Cohen’s d = 5.64, r = 0.94), but they showed an increasing trend in the control group.
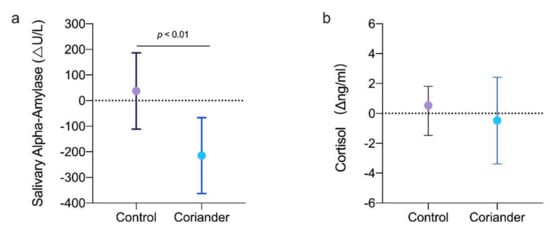

Figure 54. (a) Changes in salivary alpha-amylase (Δng/mL) between the coriander and control groups. (b) Changes in salivary cortisol (Δng/mL) between the group of coriander and control groups. Data presented as variation ± standard error (n = 13). Independent t-tests were used to identify the significant differences between the two groups.
6. Effects of Coriander on Salivary Amino Acids
We also analyzed the differences in amino acids of saliva metabolites between the control group and the coriander group (Figure 65). The results showed that the argine (p < 0.01, 95% Cl = 1.16; 3.13, Cohen’s d = 6.82, r = 0.95), proline (p < 0.01, 95% Cl = 11.37; 30.99, Cohen’s d = 6.89, r = 0.96), histidine (p < 0.05, 95% Cl = 0.01; 5.16, Cohen’s d = 3.10, r = 0.84) and taurine (p < 0.01, 95% Cl = 4.75; 15.78, Cohen’s d = 5.88, r = 0.95) contents were significantly different between two groups. In addition, the contents of these four amino acids before and after the homework were also significantly different in the coriander group, but not in the control group.
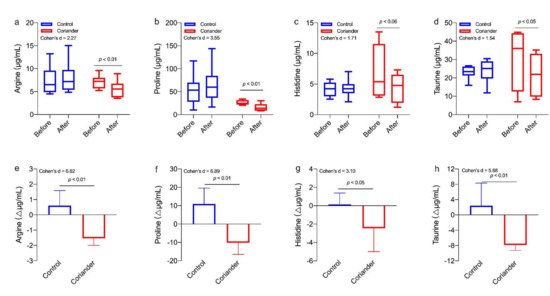

Figure 65. (a) Comparison of different salivary amino acids before and after the homework (∆µg/mL). Similar results were shown in (b–d). Paired t-tests were used to identify the significant differences before and after homework in the same group. (e) Changes in salivary metabolomics between the coriander and control groups (∆µg/mL). Similar results were shown in (f–h). Independent t-tests were used to identify the significant differences between the two groups. Data presented as variation ± standard error (n = 6).
7. Emotion Correlates with Salivary Amino Acids
The results revealed between subjective emotion and salivary metabolomics (Figure 76). The Pearson correlation analysis showed that anger was significantly and positively correlated with taurine (r = 0.58, p < 0.05), and vigor was significantly and negatively correlated with taurine (r = −0.61, p < 0.05). At the same time, self-esteem was significantly and negatively correlated with tryptophan (r = −0.62, p < 0.05) and lysine (r = −0.58, p < 0.05).
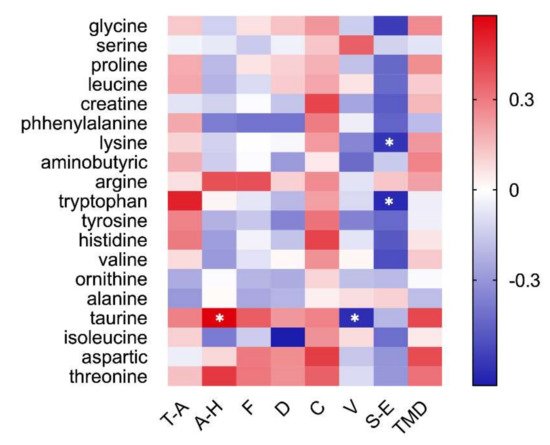

Figure 76. Correlation between subjective emotion and salivary metabolomics. Heat maps exhibit significant statistical correlation values (r). Red squares indicate positive correlation, white squares indicate no correlations, and blue squares indicates negative correlation. The deeper color means a greater correlation (* p < 0.05). Pearson’s correlation coefficient and regression analysis were performed to evaluate the connection between subjective emotional evaluation and saliva metabolome.
References
- Yoo, M.; Lee, E.-H. The Impact of Modulized Interior Landscape on Office Workers’ Psychological Wellbeing—A Pilot Study of Focused on the Office Wall. Korean Inst. Inter. Des. J. 2014, 23, 220–230.
- Peng, G.; Tian, J.; Gao, X.; Zhou, Y.; Qin, X. Research on the Pathological Mechanism and Drug Treatment Mechanism of Depression. Curr. Neuropharmacol. 2015, 13, 514–523.
- Miret, M.; Ayuso-Mateos, J.L.; Sanchez-Moreno, J.; Vieta, E. Depressive disorders and suicide: Epidemiology, risk factors, and burden. Neurosci. Biobehav. Rev. 2013, 37, 2372–2374.
- Tian, J.S.; Shi, B.Y.; Xiang, H.; Gao, S.; Qin, X.M.; Du, G.H. 1H-NMR-Based Metabonomic Studies on the Anti-Depressant Effect of Genipin in the Chronic Unpredictable Mild Stress Rat Model. PLoS ONE 2013, 8, e75721.
- Baglioni, C.; Battagliese, G.; Feige, B.; Spiegelhalder, K.; Nissen, C.; Voderholzer, U.; Lombardo, C.; Riemann, D. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J. Affect. Disord. 2011, 135, 10–19.
- Liu, D.; Gong, L.; Shen, J.; Lu, T. Discussion on the value of core self-evaluation for the prevention and treatment of depression. Diet Health 2021, 16, 102.
- Soga, M.; Gaston, K.J.; Yamaura, Y. Gardening is beneficial for health: A meta-analysis. Prev. Med. Rep. 2017, 5, 92–99.
- Song, C.; Joung, D.; Ikei, H.; Igarashi, M.; Aga, M.; Park, B.J.; Miwa, M.; Takagaki, M.; Miyazaki, Y. Physiological and psychological effects of walking on young males in urban parks in winter. J. Physiol. Anthropol. 2013, 32, 18.
- Park, B.J.; Tsunetsugu, Y.; Lee, J.; Kagawa, T.; Miyazaki, Y. Effect of the forest environment on physiological relaxation using the results of field tests at 35 sites throughout Japan. For. Med. 2012, 57–67.
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Suzuki, H.; Li, Y.J.; Wakayama, Y.; et al. Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2008, 21, 117–127.
- Sofo, A.; Sofo, A. Converting Home Spaces into Food Gardens at the Time of Covid-19 Quarantine: All the Benefits of Plants in this Difficult and Unprecedented Period. Hum. Ecol. 2020, 48, 131–139.
- Zhang, W.; Liu, H.; Li, Z.; Liu, H. Synergistic effects of edible plants with light environment on the emotion and sleep of humans in long-duration isolated environment. Life Sci. Space Res. 2020, 24, 42–49.
- Bedrosian, T.A.; Nelson, R.J. Influence of the modern light environment on mood. Mol. Psychiatry 2013, 18, 751–757.
- Duesenberg, M.; Weber, J.; Schulze, L.; Schaeuffele, C.; Roepke, S.; Hellmann-Regen, J.; Otte, C.; Wingenfeld, K. Does cortisol modulate emotion recognition and empathy? Psychoneuroendocrinology 2016, 66, 221–227.
- Bringslimark, T.; Hartig, T.; Patil, G.G. The psychological benefits of indoor plants: A critical review of the experimental literature. J. Environ. Psychol. 2009, 29, 422–433.
- Cohen-Mansfield, J.; Werner, P. Outdoor wandering parks for persons with dementia: A survey of characteristics and use. Alzheimer Dis. Assoc. Disord. 1999, 13, 109–117.
- Sahoo, S.; Brijesh, S. Anxiolytic activity of Coriandrum sativum seeds aqueous extract on chronic restraint stressed mice and effect on brain neurotransmitters. J. Funct. Foods 2020, 68, 103884.
- Bendifallah, L.; Louadi, K.; Doumandji, S. Bee fauna potential visitors of coriander flowers Coriandrum sativum L. (Apiaceae) in the Mitidja area (Algeria). J. Apic. Sci. 2013, 57, 59–70.
- Adams, M.; Schneider, S.V.; Kluge, M.; Kessler, M.; Hamburger, M. Epilepsy in the Renaissance: A survey of remedies from 16th and 17th century German herbals. J. Ethnopharmacol. 2012, 143, 1–13.
- Padalia, K.; Bargali, K.; Bargali, S.S. How does traditional home-gardens support ethnomedicinal values in Kumaun Himalayan Bhabhar belt, India? African J. Tradit. Complement. Altern. Med. 2015, 12, 100–112.
- Laribi, B.; Kouki, K.; M’Hamdi, M.; Bettaieb, T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia 2015, 103, 9–26.
- Yuan, R.; Liu, Z.; Zhao, J.; Wang, Q.-Q.; Zuo, A.; Huang, L.; Gao, H.; Xu, Q.; Khan, I.A.; Yang, S. Novel compounds in fruits of coriander (Coşkuner & Karababa) with anti-inflammatory activity. J. Funct. Foods 2020, 73, 104145.
More
