Aquilaria sinensis (Lour.) Gilg is the resin-containing wood of the Aquilaria. Agarwood is a traditional Chinese medicine included in the 2020 edition of Chinese Pharmacopoeia. The main phytochemicals of agarwood include terpenoids, dominated by sesquiterpenes. For centuries, terpenoids have been used in traditional Chinese medicine and have been shown to possess various pharmacological properties, including bacteriostatic, antibacterial, sedation, analgesia, anti-inflammation, anti-asthmatic, hypoglycemic, antidepressant, and many others. Alongside biological activity screening, phytochemical advances and pharmacological research have also made certain progress.
- agarwood
- chemistry
- medicinal plant
- pharmacological effects
- phytochemicals
1. Introduction
|
Species |
Origin |
References |
|---|---|---|
|
Aquilaria malaccensis Lam. |
India, Myanmar, Malaysia, Indonesia, Philippines |
3.2. Phytochemicals
|
Name |
Contents (%) |
Chemical Structure |
Reference |
|---|
|
Name |
Chemical Structure |
Reference |
|---|
[ | |||
|
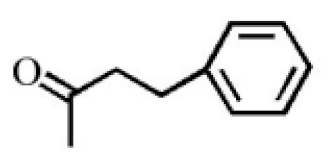
Benzylacetone |
0.95 | ||
] | [ | ||
|
|
[20] |
|
|
|
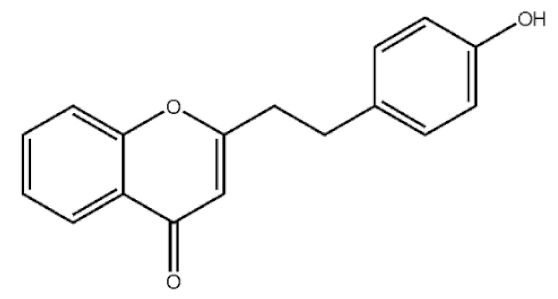
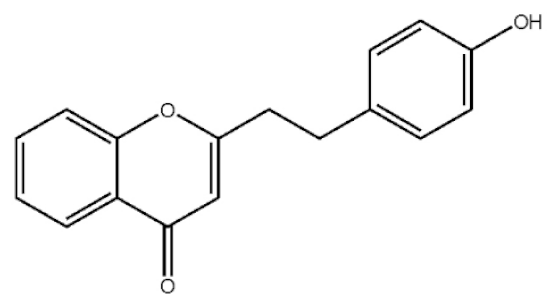
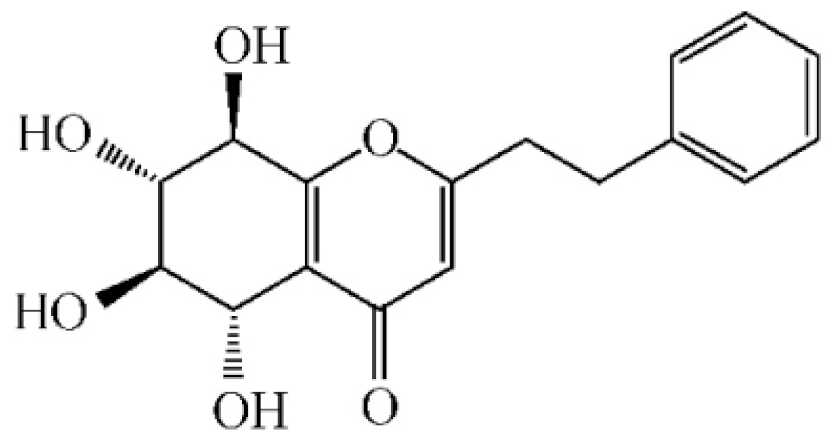
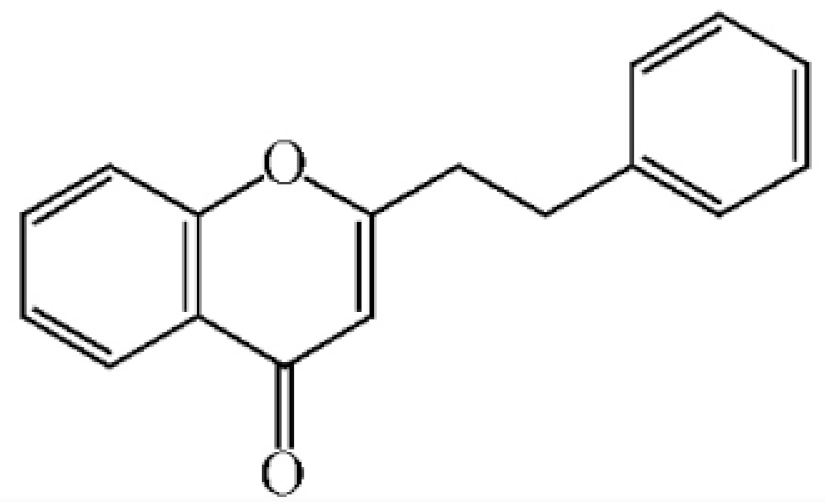
2-[2-(4-Hydroxyphenyl)ethyl]chromone |
|
|
|
|
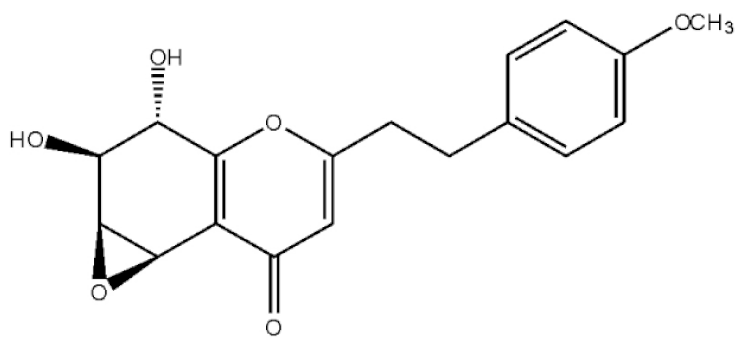
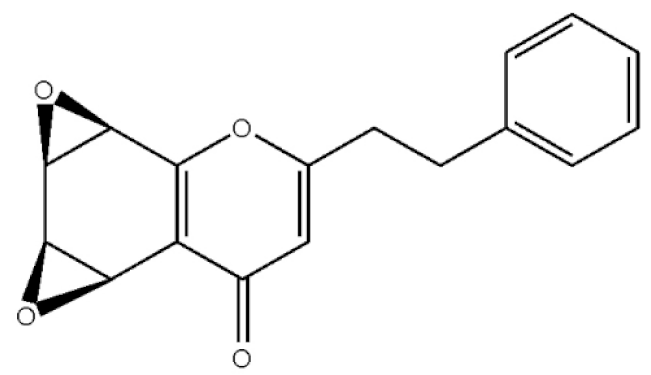
Rel-(1AR,2R,3R,7bS)-1a,2,3,7b-Tetrahydro-2,3-hidydroxy-5[2-(4-methoxyphenyl)ethy]-7H-oxireno[f][1]benzophran-7-one |
|
|
Oxidoagarchromones A |
|
[ | |
] | |
3.3. Pharmacological Uses
|
Pharmacological Action |
Active Substance |
Action Mechanism |
|---|
|
Morphological Feature |
Grade |
|||
|---|---|---|---|---|
|
A |
B |
C |
||
|
Antibacterial and bacteriostatic |
Sesquiterpenes | [21] |
Its antibacterial mechanism can induce cell apoptosis through the process of nuclear condensation and cleavage. |
|
|
Aquilaria sinensis (Lour.) Gilg |
||||
|
2,4-Di-tert-butylphenol | ||||
China |
[7][ | |||
|
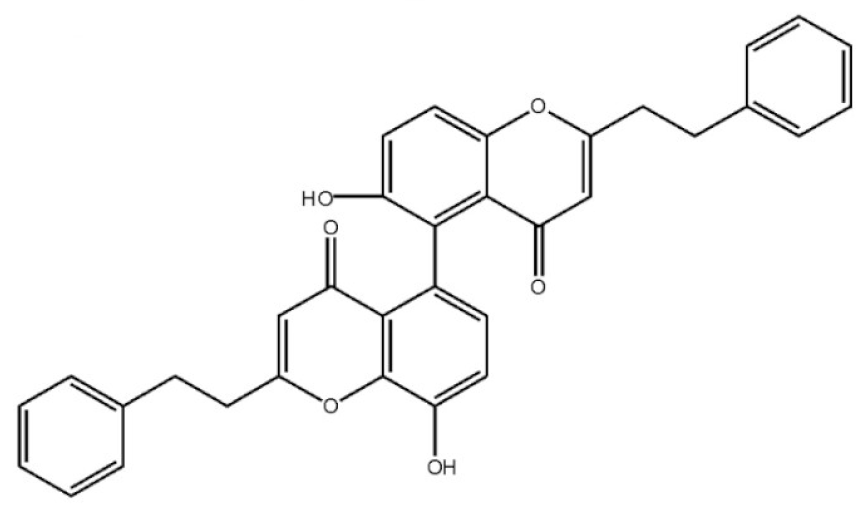
Anti-tumor6,8′-Dihydroxy-2-2′-bis(2-phenylethyl)-4H,4′H-5,5′-bichromone-4,4′-dione |
|
|||
|
Agarotetrol | ||||
|
Color |
Dark brown or khaki with a small number of white spots |
Yellowish or reddish-brown with more white spots |
Khaki or yellowish-brown with a large number of white spots |
|
|
4.15 |
||||
|
Density of resin |
Sesquiterpene, chromone, and triterpene |
Very dense and compact, sink in water when soaked | ||
] | ||||
Its mechanism may be related to the induction of apoptosis through nuclear condensation and breakage, including the destruction of mitochondrial membrane potential. |
full-floating on water | |||
Dense and less compact, half-sinkage in water | ||||
|
Aquilaria microcarpa Baill |
Indonesia |
|||
|
Sedation, analgesia, and anti-inflammation | ||||
[ | ||||
Sesquiterpene and chromone | ||||
] | [9 | |||
|
| ||||
Light and not dense, | The mechanism of analgesia and sedation may be related to the regulation of gene expression of GABAA receptor, enhancement of GABAA receptor function, and promotion of Cl−1] | |||
|
Weight |
Hard texture, brittle, and not hollowed | influx, and the anti-inflammatory mechanism may be related to the inhibition of granulocyte respiratory burst, inhibition of pro-inflammatory cytokines (IL-1 β, IL-6, and TNF- α), and decrease in lipid peroxidation (MDA). |
The texture is a little hard, a little brittle, and slightly hollow |
Loose texture, not brittle, and hollow |
|
Aquilaria apiculata Merr |
||||
|
Relieving cough and relieving asthma | ||||
Philippines |
||||
Terpenoid/chromone | ||||
[ | ] |
|||
It is speculated that the mechanism by which agarwood relieves asthma may be related to anti-inflammation, anti-apoptosis, improvement of pathological changes of lung and an intestinal tract, abnormal function, and balance of immunity. | ||||
|
Aroma |
Strong odor, feel sweet and cool. |
Less potent odor, feel sweet and slightly spicy |
The aroma is light, feel slightly sweet and salty |
|
|
Aquilaria baillonii Pierre ex Lecomte |
||||
|
Antidepressant | ||||
Cambodia, Thailand, Laos, Vietnam |
||||
Diterpene |
||||
|
Sense of oiliness | The mechanism may be related to the inhibition of the corticotropin-releasing factor (CRF) gene expression and the hyperactivity of the hypothalamus–pituitary–adrenal (HPA) axis, as well as the inhibition of corticotropin receptor gene transcription and protein expression in the cerebral cortex and hippocampus. |
Have a strong sense of oiliness | ||
|
Aquilaria banaensis P.H.H6 |
||||
| ||||
Have a strong sense of oiliness |
The sense of oiliness is weak |
Anti-oxidation and anti-aging | ||
Vietnam |
||||
[ | ||||
| [ | 22 | ][23] | ||
Flavonoids and sesquiterpenes |
The mechanism may be related to the regulation of reactive oxygen clusters and proinflammatory cytokines by microglia and the release of stress hormones. |
|||
|
Aquilaria beccariana Tiegh |
Indonesia |
|||
|
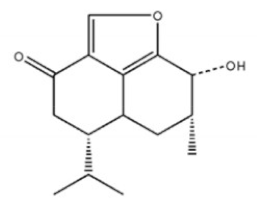
Isoagarotetrol | ||||
[ | ][7] |
|||
|
||||
|
Aquilaria citrinicarpa (Elmer) Hallier f. |
Philippines |
|||
|
2-(2-phenylethyl) chromone |
| |||
] | ||||
|
Aquilaria cumingiana (Decne) Ridl |
Malaysia |
[8] |
||
|
2-[2-(4-methoxyphenyl) ethyl] chromone |
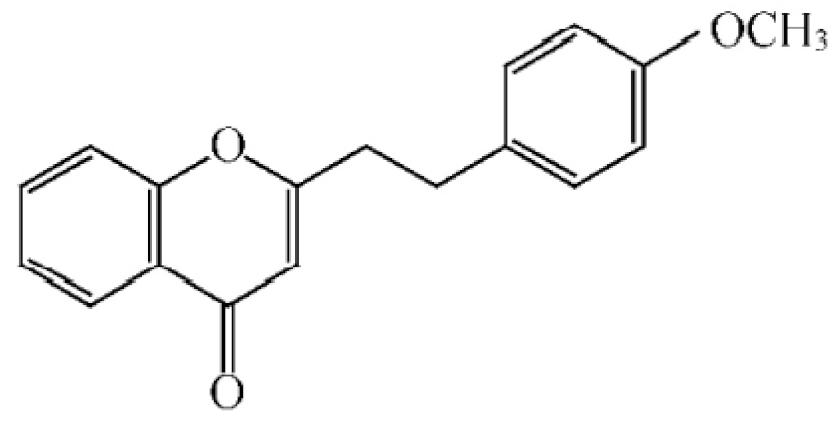 |
|||
Aquilaria khasiana Hallier f. |
India |
|||
|
Aquilaria apiculata Merr |
Philippines |
|||
|
Aquilaria parvifolia (Quisumb) Ding Hon |
Philippines |
|||
|
Aquilaria rostrata Ridl |
Malaysia |
|||
|
Aquilaria rugosa Kiet Kessler |
Vietnam |
[8] |
||
|
Aquilaria subintegra Ding Hon |
Thailand |
|||
|
Aquilaria urdanetensis (Elmer) Hallier f. |
Philippines |
|||
|
Aquilarla yunnanensis S.C. Huang |
China |
|||
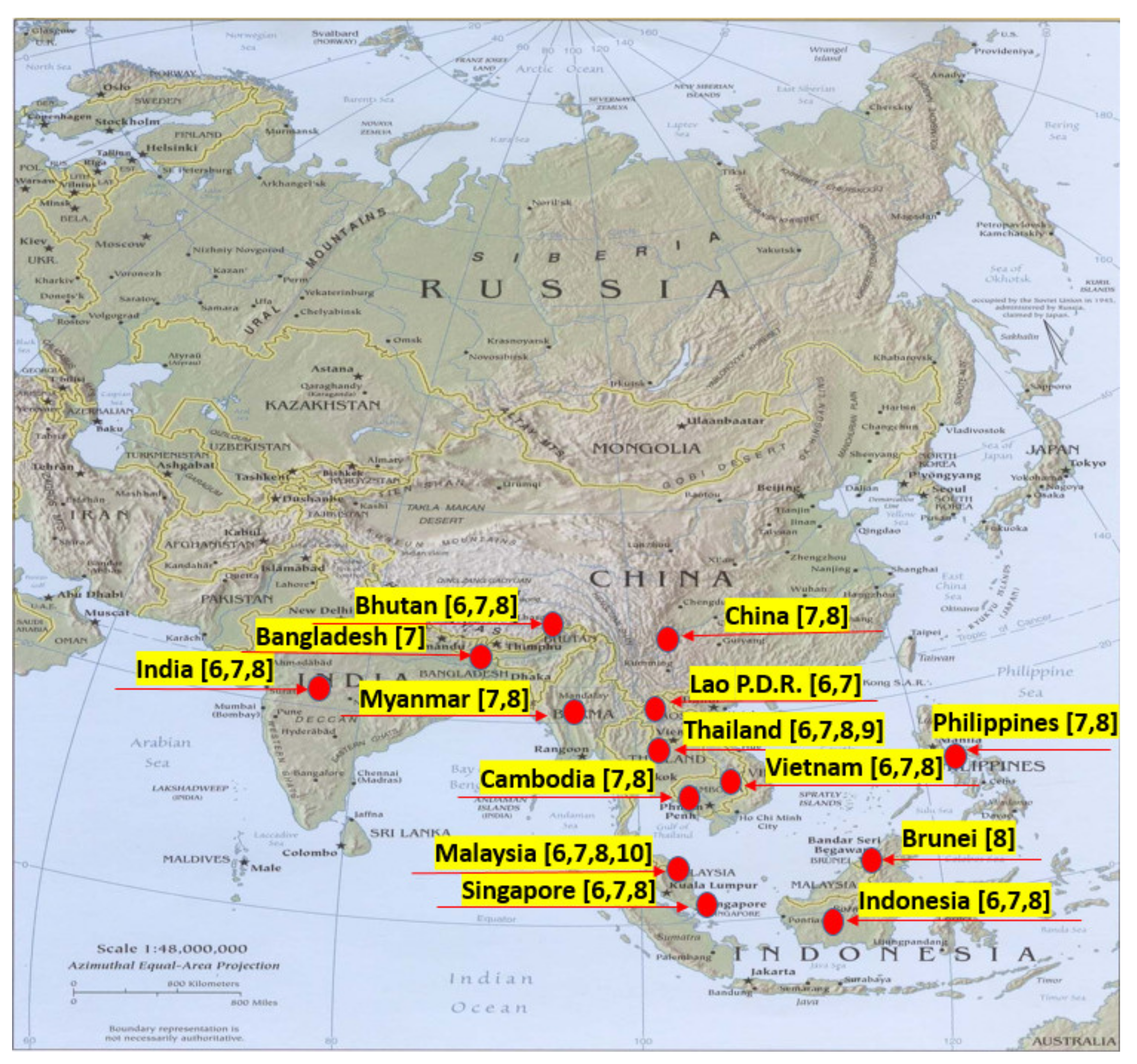
2. Economic Value and Aquilaria Species Distribution
Economic Value
3. Aquilaria Identification Methods and Phytochemicals
3.1. Identification Methods
|
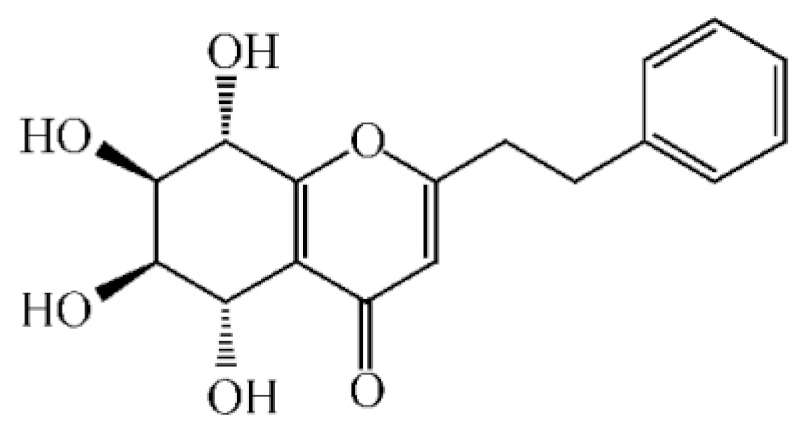
5,6,7,8,-Tetrahydroxy-5,6,7,8-tetrahydro-2-[2-(4-methoxyphenyl)ethy]-chromone | |||
|
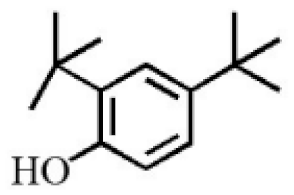
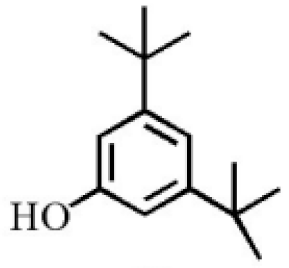
3,5-Di-tert- butylphenol |
2.70 |
|
|
|
4-Methoxyphenylacetone |
0.95 |
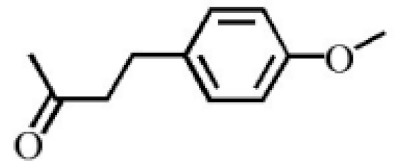 |
Cardiovascular system | ||
Chromone | ||
The mechanism may inhibit cardiomyocyte apoptosis after ischemia/reperfusion by regulating B lymphocyte tumor-2 gene (Bcl-2) and to down-regulating rabbit anti-human monoclonal antibody (Bax). |
|
Name |
Chemical Structure |
Reference |
|---|---|---|
|
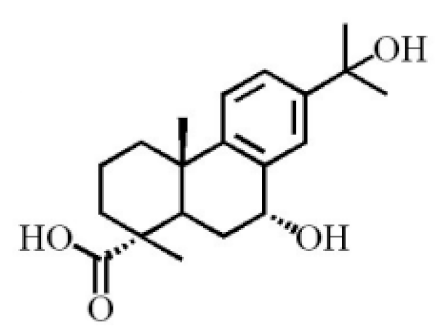
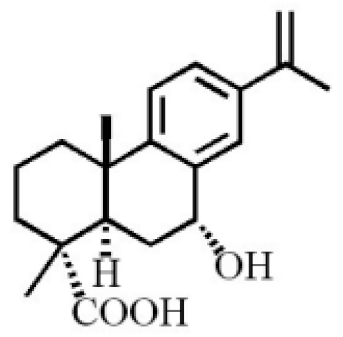
7α,15-Dihydroxydehydroabietic acid |
|
[24] |
|
Methyl 7-oxodehydroabietate |
|
|
|
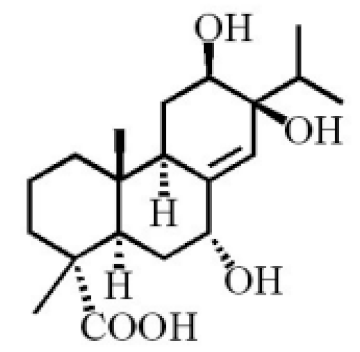
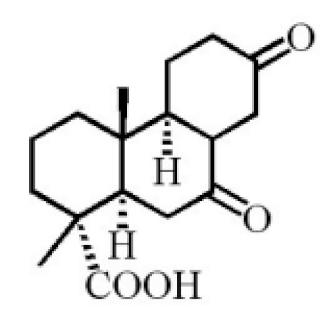
7α-hydroxypodocarpen-8(14)-en-13-on-18-oic acid |
|
|
|
18-norpimara-8(14),15-dien-4αα-ol |
|
|
|
18-norpimara-8(14), and 15-dien-4α-ol |
|
|
|
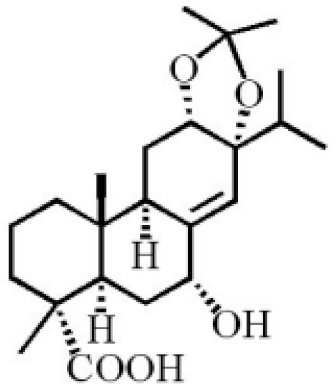
7α, 12α, 13α-trihydroxyabiet-8(14)-en-18-oic acid acetonide |
|
[25] |
|
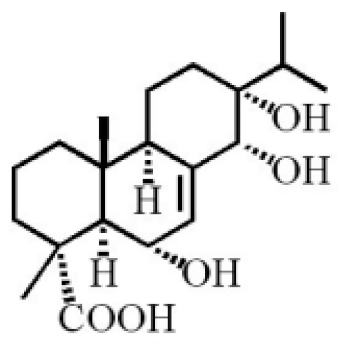
6α, 13α, 14α-trihydroxyabiet-7-en-18-oic acid |
|
|
|
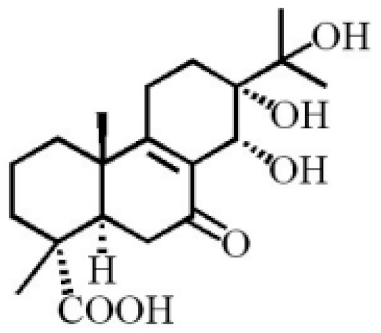
13α, 14α, 15-trihydroxy-7-oxoabiet-8-en-18-oic acid |
|
|
|
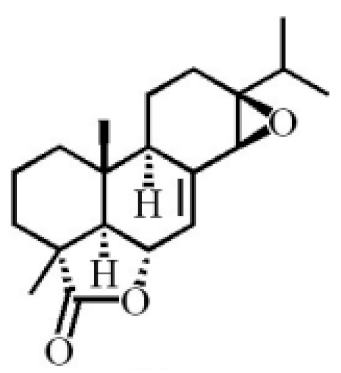
13β, 14β-epoxyabiet-7-en-18, 6α-olide |
|
|
|
7α, 12β, 13β-trihydroxyabiet-8(14)-en-18-oic acid |
|
|
|
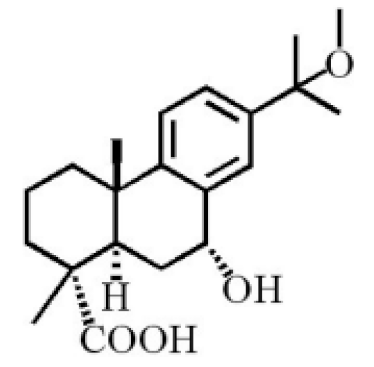
7α-hydroxyabieta-15-methoxy-8,11,13-trien18-oic acid |
|
|
|
7α-hydroxyabieta-15-methoxy-8,11,13-trien18-oic acid |
|
|
|
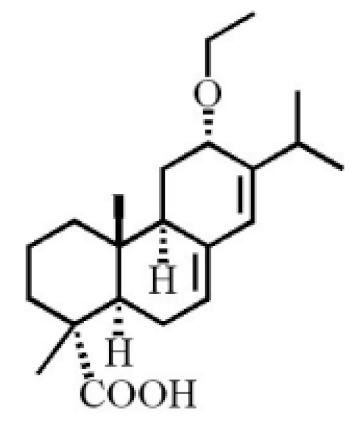
12α-ethoxyabieta-7,13-dien-18-oic acid |
|
|
|
7,13-dioxopodocarpan-18-oic acid |
|
|
|
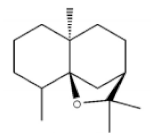
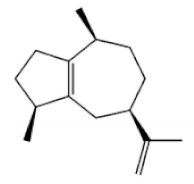
α-Agarofuran |
|
[26] |
|
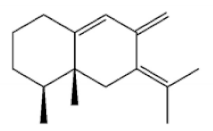
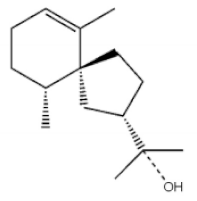
(5S,7S,10S)-(-)Selina-3,11-dien-9-one |
|
|
|
(+)-(4S,5R)-Dihydrokaranone |
|
|
|
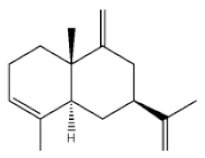
α-Guaiene |
|
|
|
Agarospirol |
|
|
|
8-β-H-Dihydrogmelofuran |
|
|
|
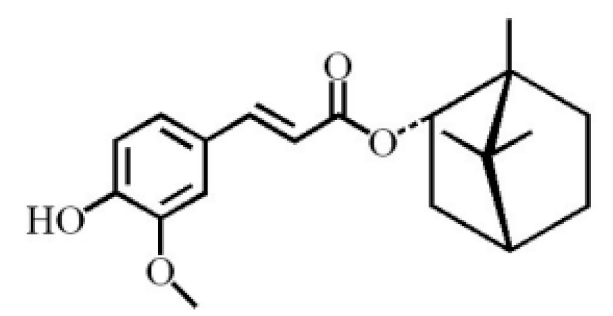
(-)-bornyl ferulate |
|
3.4. Grading System for Agarwood Identification
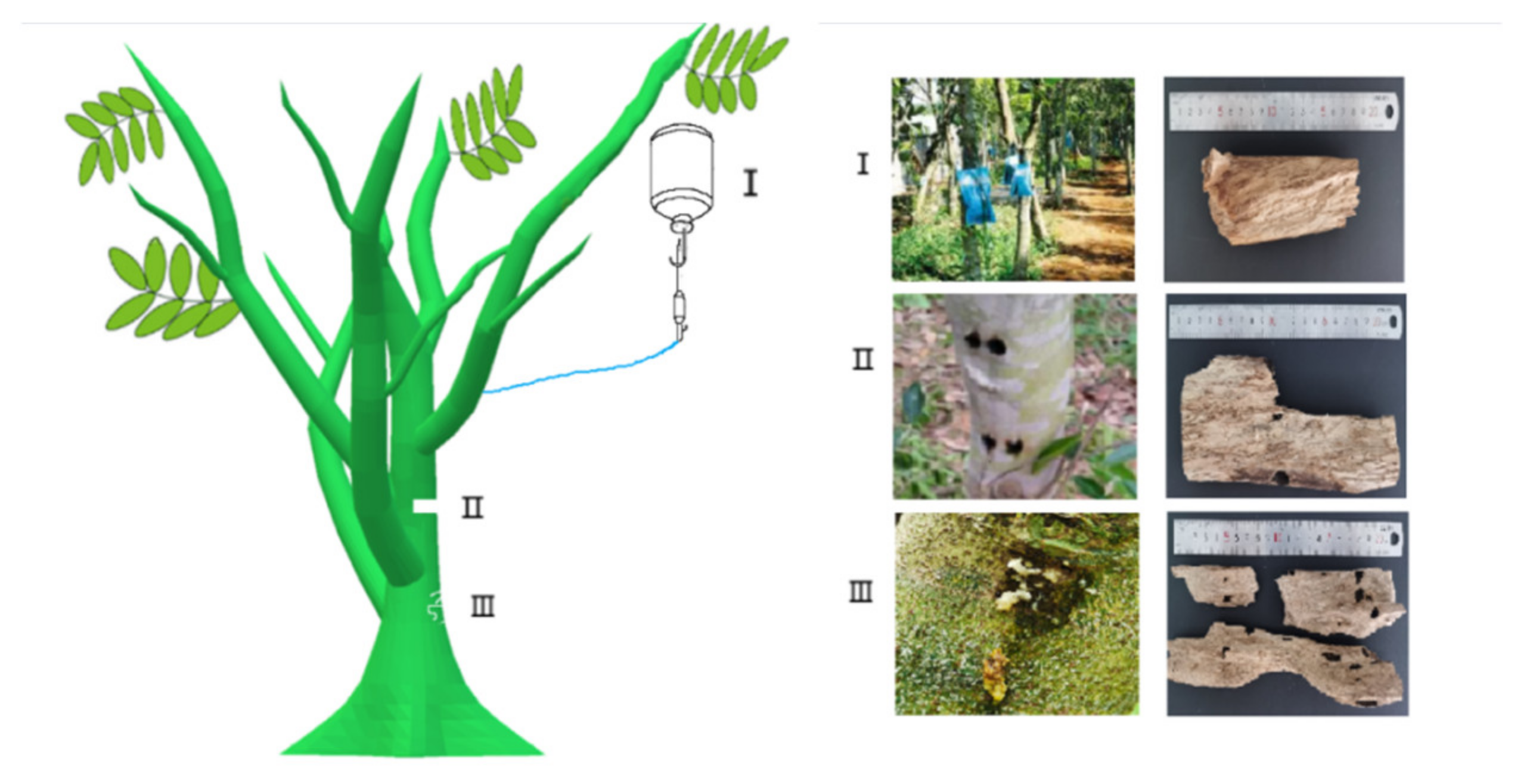
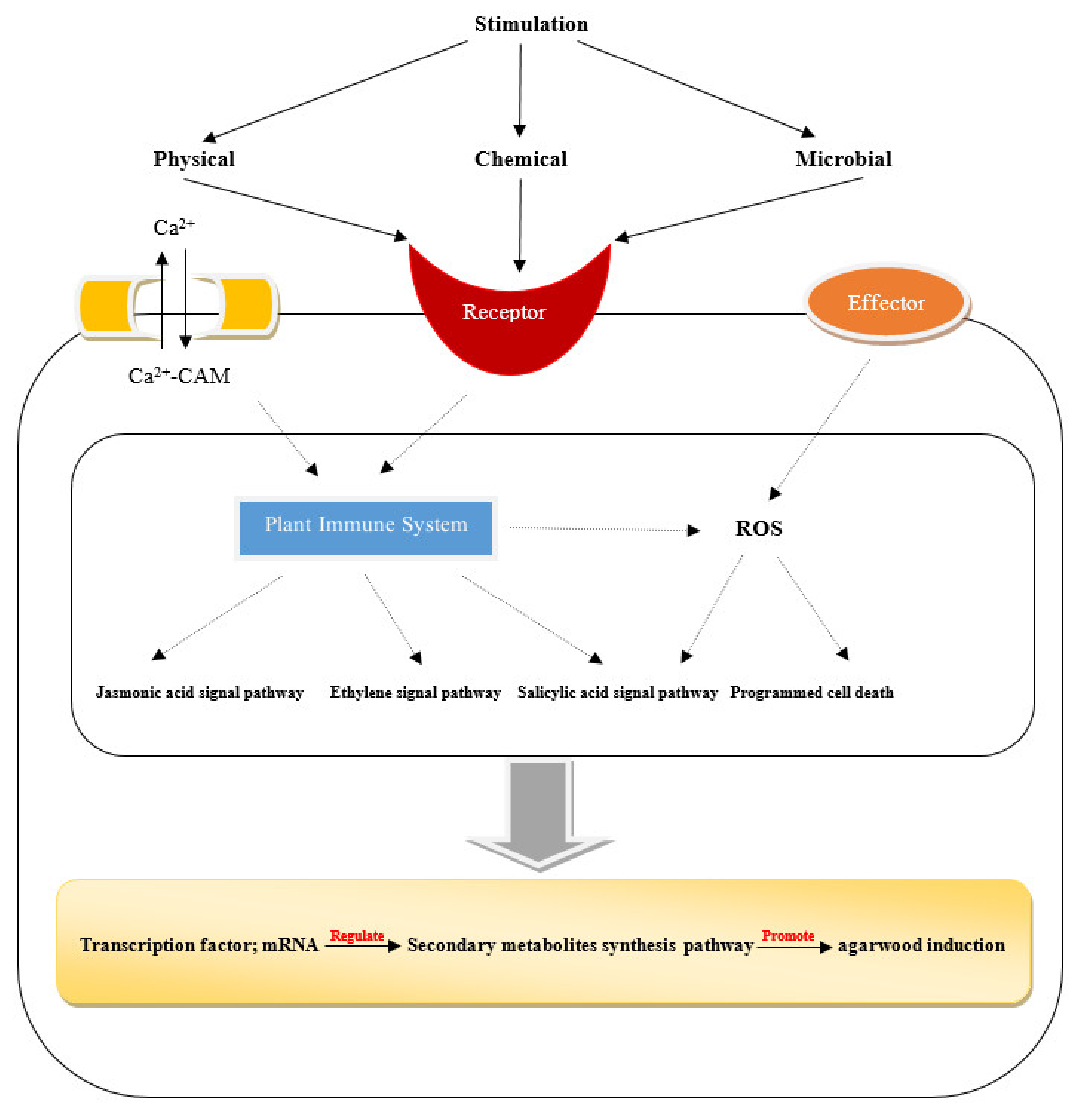
3.5. Agarwood Induction Technique
3.5.1. Natural Induction
3.5.2. Artificial Induction
References
- Chinese Pharmacopoeia. National Pharmacopoeia Committee. 2020. Available online: http://wp.chp.org.cn/front/chpint/en/ (accessed on 19 December 2021).
- Liu, Y.Y.; Wei, J.H.; Gao, Z.H.; Zhang, Z. A review of quality assessment and grading for agarwood. Chin. Herb. Med. 2017, 9, 22–30.
- Kumeta, Y.; Ito, M. Characterization of α-humulene synthases responsible for the production of sesquiterpenes induced by methyl jasmonate in Aquilaria cell culture. J. Nat. Med. 2016, 70, 452–459.
- Liu, Y.Y.; Chen, D.L.; Wei, J.H.; Feng, J.; Zhang, Z.; Yang, Y.; Zheng, W. Four new 2-(2-phenylethyl) chromone derivatives from Chinese agarwood produced via the whole-tree agarwood-inducing technique. Molecules 2016, 21, 1433.
- Yumi, Z.; Has-Yun, H.; Kerr, P.G.; Phirdaous, A.; Hamzah, M.S. Aquilaria spp. (agarwood) as source of health beneficial compounds: A review of traditional use, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 189.
- Barden, A.; Anak, N.A.; Mulliken, T.; Song, M. Heart of the matter: Agarwood use and trade and CITES implementation for Aquilaria malaccensis. Traffic Network Rep. 2000, 46, 17–18.
- Gärdenfors, U.; Hilton Taylor, C.; Mace, G.M.; Rodriguez, J. The application of IUCN red list criteria at regional levels. Conserv. Biol. 2001, 15, 1206–1212.
- Germplasm Resources Information Network Taxonomy for Plant. Available online: https://trade.cites.org/# (accessed on 12 December 2021).
- Hsuan, K. The Concise Flora of Singapore. Gymnosperms and Dicotyledons; University Press: Singapore, 1990.
- Parris, B.S. Flora of Peninsular Malaysia; Forest Research Institute: Kuala Lumpur, Malaysia, 2010.
- Wong, Y.F.; Chin, S.T.; Perlmutter, P.; Marriott, P.J. Evaluation of comprehensive two-dimensional gas chromatography with accurate mass time-of-flight mass spectrometry for the metabolic profiling of plant–fungus interaction in Aquilaria malaccensis. J. Chromatogr. 2015, 1387, 104–115.
- Ismail, N.; Ali, N.A.M.; Jamil, M.; Rahiman, M.H.F.; Tajuddin, S.N.; Taib, M.N. A review study of agarwood oil and its quality analysis. J. Teknol. 2014, 68, 37–42.
- Sen, S.; Talukdar, N.C.; Khan, M. A simple metabolite profiling approach reveals critical biomolecular linkages in fragrant agarwood oil production from Aquilaria malaccensis–a traditional agro-based industry in North East India. Curr. Sci. 2015, 108, 63–71.
- Ishihara, M.; Tsuneya, T.; Uneyama, K. Components of the agarwood smoke on heating. J Essent. Oil Res. 1993, 5, 419–423.
- Ishihara, M.; Tsuneya, T.; Uneyama, K. Components of the volatile concentrate of agarwood. J. Essent. Oil Res. 1993, 5, 283–289.
- Du, H.f.; Xiong, L.y.; Lin, L.; Sshuai, O.; Qi, L.k. Simultaneous determination of five kinds of chromone in Aguilariae lignum resinatum by HPLC. CTMFJ 2015, 20, 87–90.
- Zou, M.M.; Xia, Z.Q.; Lu, C.; Wang, H.; Ji, J.; Wang, W.J. Genetic diversity and differentiation of Aquilaria sinensis (Lour.) Gilg revealed by ISSR and SRAP markers. Crop Sci. 2012, 52, 2304–2313.
- Niu, X.; Ji, K.; Lu, G. Preliminary studies on identification of Aquilaria sinensis (L.) Gilg by the PCR product of rDNA ITS sequencing. Gd. Agric. Sci. 2010, 37, 167–169.
- Shen, Y.; Tan, X.; Zhao, X.; Pang, Q.; Zhao, S.J. Pharmacy, Ribosomal DNA ITS sequence analysis of Aquilaria sinensis from different geographical origin in China. Chin. J. Trad. Chin. Med. Pharm. 2009, 24, 539–541.
- Mei, W.L.; Zeng, Y.B.; Liu, J.; Dai, F. GC-MS analysis of volatile constituents from five different kinds of Chinese eaglewood. J. Chin. Med. Mat. 2007, 30, 551–555.
- Yang, J.J. Study on quality evaluation method of agarwood from China. Master’s Thesis, Hainan University, Haikou, China, 2015. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201702&filename=1015592278.nh&uniplatform=NZKPT&v=UxkWd_b6Efird7uOwyv1LxhWn2np-jaCL5kGrHJG0gB9s3ToeGtfVufTgjYZpRhf (accessed on 19 December 2021).
- Shimada, Y.; Tominaga, T.; Konishi, T.; Kiyosawa, S. Studies on the agarwood (Jinko). I. Structures of 2-(2-phenylethyl) chromone derivatives. Chem. Pharm. Bull. 1982, 30, 3791–3795.
- Shimada, Y.; Konishi, T.; Kiyosawa, S.; Nishi, M.; Miyahara, K.; Kawasaki, T. Studies on the agalwood (Jinko). IV.: Structures of 2-(2-Phenylethyl) chromone derivatives, agarotetrol and isoagarotetrol. Chem. Pharm. Bull. 1986, 34, 2766–2773.
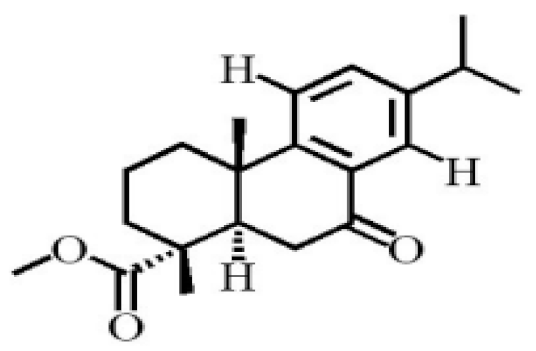
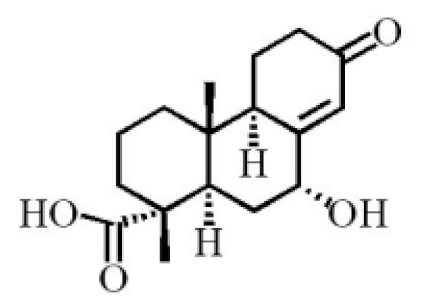
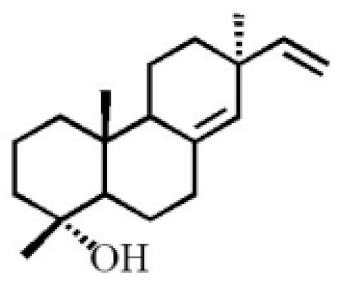
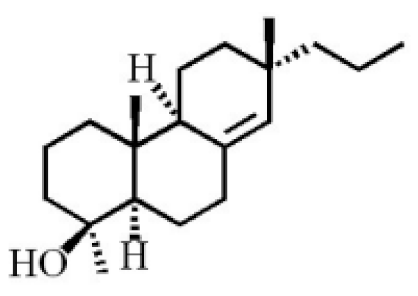
- Yang, L.; Qiao, L.R.; Xie, D.; Dai, J.G.; Guo, S.X. Diterpenoids from Chinese eaglewood. Chin. J. Chin. Mat. Med. 2011, 36, 2088–2091.
- Yang, L.; Qiao, L.R.; Ji, C.X.; Xie, D.; Gong, N.B.; Lu, Y.; Zhang, J.J.; Dai, J.G.; Guo, S.X. Antidepressant abietane diterpenoids from Chinese eaglewood. J. Nat. Prod. 2013, 76, 216–222.
- Sukkaew, S.; Pripdeevech, P.; Thongpoon, C.; Machan, T.; Wongchuphan, R. Volatile constituents of murraya koenigii fresh leaves using headspace solid phase microextraction–gas chromatography–mass spectrometry. Nat. Prod. Commun. 2014, 9, 1783–1786.
- Chen, H.Q.; Wei, J.H.; Yang, J.S.; Zhang, Z.; Yang, Y.; Gao, Z.H.; Sui, C.; Gong, B. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem. Biodivers. 2012, 9, 236–250.
- Xie, B.; Luo, H.; Huang, X.; Huang, F.; Zhang, Q.; Wu, X.; Zhou, X.; Wu, H. Pharmacokinetic studies of six major 2-(2-phenylethyl) chromones in rat plasma using ultra high performance liquid chromatography with tandem mass spectrometry after oral administration of agarwood ethanol extract. J. Separ. Sci. 2021, 44, 2418–2426.
- Ismail, N.; Ali, N.A.M.; Jamil, M.; Rahiman, M.H.F.; Taib, M.N.; Tajuddin, S.N. Major volatile chemical compounds of agarwood oils from Malaysia based on z-score technique. J. Chem. Nat. Compd. 2015, 51, 776–779.
- Chen, X.Y.; Huang, Y.Q. Grey relational analysis of volatile components and anti-tumor activity of agarwood. Proprietary Chin. Med. 2018, 40, 224–227.
- Zhou, X.; Fan, Y.; Lei, Z.; Pan, Q.; Zhong, Z.; Liu, D.; Zhang, W.; Gao, X. Correlation between agaropiric acid and extract contents in artificial Aquilariae lignum resinatum. Chin. J. Exp. Tradit. Med. Form. 2016, 22, 55–59.
- Zhao, Y.; Fang, Z.J. Quality assessment of Chinese eaglewood by the technique of Aquilaria sinensis quintana making incense and the determination of benzyl acetone. J. Gd. Pham. Univ. 2013, 29, 253–257.