You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Antonio Giuliano.
Laboratory rodents are the most common animal models used in preclinical cancer research. Companion animals with naturally occurring cancers are an under-utilized natural model for the development of new anti-cancer drugs. Dogs and cats develop several types of cancers that resemble those arising in humans with similar clinical and histopathological features and often with similar molecular and genetic backgrounds. Exposure to environmental carcinogens, including air, food and water are also common between people and their pets. Dogs and cats are a unique model that could be integrated between the preclinical laboratory animal model and human clinical trials.
- companion animal model
- feline oral squamous cell carcinoma
- head and neck carcinoma
- immunotherapy
- mucosal oral melanoma
1. Spontaneously Occurring Cancers in Companion Animals Represent a Unique Model for Human Cancers
The main goal of cancer research is to find new diagnostic and therapeutic anti-cancer strategies. Preclinical cancer research is mainly based on laboratory animal models with most of the studies performed on tumors grown in rodents [1]. Laboratory in vitro and in vivo studies have been and remain an essential first step in cancer research. The main goal of preclinical research is to assess the toxicity and efficacy of new drugs prior to conducting human trials [2]. Despite the common use of mice and other rodents in preclinical research, these models are poorly predictive of efficacy in human clinical trials [3,4][3][4]. Despite many successful laboratory animal studies, only 5–8% of new anticancer drugs are eventually approved for clinical use [5,6][5][6].
The lack of success in preclinical laboratory mice studies, both in xenograft and genetically engineered mice, could result from the failure to accurately reproduce the biological behavior and genetic and molecular background of the artificially induced cancer compared to a spontaneous human tumor, the inability to examine the specific tumor microenvironment and host characteristics, and a lack of the complexity and heterogenicity of naturally occurring cancers [6,7][6][7]. Experiments conducted in caged laboratory animals can be affected by high levels of stress that could affect the response to investigational drugs and immunotherapy. A lack of well-defined best practice protocols for the testing, treatment and procedures performed in laboratory animals compared to human clinical trials could also introduce bias [8,9][8][9].
Companion animals represent a unique model for human cancer for various reasons. Dogs and cats, unlike laboratory rodents, develop naturally occurring cancers that closely mimic the heterogenous nature of human tumors [10,11][10][11]. Many cancers arising in dogs and cats have similar clinical signs, appearances, and biological behavior to human cancers. Microscopic appearance and genetic and molecular background are also very similar in many types of cancers in dogs, cats, and people. The outbred characteristic in dogs, compared to studies in inbred laboratory animals, provides a background of genetic diversity that more closely parallels that of humans [11,12][11][12]. In addition, compared with the murine genome, the canine genome more closely resembles the human genome [12,13][12][13].
Cancer in pets, as in people, is one of the leading causes of death [13,14][13][14]. The life span of dogs and cats has increased in recent decades and now the incidence of cancer in dogs exceeds that of people, with around 40–50% of dogs older than 10 years dying of cancer [15,16][15][16]. Companion animals share all their lives with their owners, including the environmental and socioeconomic factors that predispose to cancer development [17]. For example, obesity is considered one of the leading factors associate with increased cancer incidence, morbidity, and mortality in people [18]. Obesity also affects dogs and cats. Around 50% of dogs are considered overweight [19] and obese dogs are also more likely to have an owner that is obese [20]. Companion animals and people are exposed to similar environmental risks factors, toxins, and carcinogens such as air pollution or pesticides in food and water [21]. Spontaneous companion animal tumor models are likely to mimic the intricate and complex metabolic, genetic, and epigenetic alterations that are associated with cancer in people [22].
Companion animals have a larger body size compared to rodents, allowing for easier and more frequent blood sampling for longitudinal assessment of drug efficacy/toxicity. Furthermore, the collection of larger biopsy samples compared to those of mice can be advantageous when multiple analyses are required [23]. Identical imaging modalities can be applied to cancers in animals and humans (x-ray, CT, and MRI scan) so that any findings can be easily interpreted and compared. Responses to chemotherapy and drug toxicity in people is more comparable to those of companion animals than mice, and similar drugs are used to treat cancer in people and in pets [23]. As an example, the CHOP chemotherapy protocol involving the use of cyclophosphamide, doxorubicin, vincristine, and prednisolone is used as a standard of care for the treatment of the most common type of lymphoma, diffuse large cell lymphoma (DLCL), in both dogs and human patients. Response rates and outcomes for some cancers (approximately one year survival in dogs translates to five years in people) are also comparable [11]. However, many chemotherapy drugs are used at lower doses in pets compared to people as the main goal of treatment in veterinary oncology is to improve quality of life rather than attempting to achieve a cure [24]. While conventional chemotherapy is used at lower doses in pets to avoid severe adverse events, target therapies and immunotherapies can often be used at similar doses in pets as in human patients [25].
In the new era of cancer immunotherapy, companion animal models could play a key role in the testing and development of new treatment in people. The intricate crosstalk between the immune system and cancer is very difficult to replicate in artificially induced tumors in laboratory rodents. Dogs are exposed to a multitude of antigenic stimuli across their lifespan, including pathogenic and nonpathogenic bacteria, viruses, and parasites. Considering the large exposure of the intestine to numerous microbial antigens, it is not surprising that the intestinal microbiome can influence cancer growth and response to immunotherapy [26,27][26][27]. Dogs have a naturally developed intestinal microbiome that regulates the complex response to antigenic stimulation and diseases. Dogs are likely to respond to immunotherapy similarly to people, compared to laboratory rodents kept in disease free or minimal-disease conditions. Evaluation of the response to immunotherapy treatments and the longitudinal assessment of immune response parameters/biomarkers and side effects would be easier in companion animal than mice due to the easier collection of larger blood samples. Numerous studies in pets have assessed the relationship between cancer and the immune system; increased numbers of immunosuppressive regulatory T-cells (T-reg) have been found in various cancer types in dogs [28,29,30][28][29][30]. Markers of potential response to immunotherapy such as programmed cell death 1 (PD-1) and its ligand, programmed cell death ligand 1 (PD-L1), have also been found to be overexpressed in cancer cells and cancer infiltrating lymphocytes in oral melanoma, lymphoma, osteosarcoma, and urothelial carcinoma in dogs [31,32,33,34,35][31][32][33][34][35]. The first cancer immunotherapy vaccine has been successfully used in dogs with locally controlled oral melanoma and is now conditionally licensed in the USA [36,37][36][37]. Another new promising recombinant attenuated listeria monocytogenes vaccine expressing a chimeric human HER2 for HER-2+ osteosarcoma showed safety and efficacy in dogs and further studies are ongoing [38].
In recent years, knowledge in veterinary medicine has grown significantly. Veterinarians can specialize in various medical disciplines including companion animal oncology. Qualified veterinary oncologists, like human oncologists, perform advanced treatments and follow best practice guidelines in performing clinical trials in pets. Veterinary oncologists, similarly to their medical colleagues, conduct clinical trials in dogs and cats with a strict standardized criteria for assessing grade of toxicity and tumor response [39[39][40],40], potentially offering preclinical data that are more precise, reliable, and more likely to be translated to successful human trials than the rodent models. Dogs and cats have a short life span and cancer progresses relatively quicker than in people, allowing for a more rapid collection of end-point data such as disease-free interval (DFI) and median survival time (MST) with significantly reduced cost compared to human clinical trials [11].
Regulatory policies involving companion animal clinical trials are not always well defined and clear, but in general, the regulations are more flexible than in human clinical trials [41]. As there are no clear international guidelines, rules often vary in different countries [41]. However, it is often for the institution involved in the companion animal trial to set the rules when clear guidelines are not available [42]. Clear and detailed owner consent forms and ethical approval from the institution involved in the study are usually the main requirements to perform a companion animal clinical trial in most countries [42,43][42][43].
Despite the advantages of using companion animal models, there are few limitations that need to be considered. The cancer heterogenicity of spontaneous companion animal models is an advantage, but also a disadvantage. When specific genetic/pathway alterations need to be studied, a more homogeneous and less diverse genetic background is preferable. Another limitation is cost; despite pet trials being cheaper than their human counterparts, they are still more expensive and more time consuming than rodent preclinical studies [23]. Owner willingness to be enrolled in investigational studies and compliance with the terms and conditions of the trial are other potential disadvantages. Furthermore, an important factor that needs to be considered in pet trials is euthanasia. In many western countries, euthanasia in pets is widely recognized as a humane way to end life and so the subjective and personal decision of the owner to euthanize their pets could affect standardization of the MST. Despite the similarities of cancers in people and companion animals, there are species-specific differences in incidence as well as biological and clinical behavior that needs to be considered before choosing a specific companion animal cancer model. Feline oral squamous cell carcinoma and canine oral melanoma have previously been considered a good model for people suffering from head and neck carcinoma and mucosal melanoma, respectively [17,44][17][44]. An updated summary of the findings in these two types of tumors for translational cancer research, including new possible translational immunotherapies, will be discussed in more detail.
2. Feline Oral Squamous Cell Carcinoma in Cats as a Model of Head and Neck Squamous Cell Carcinoma in People
Incidence, Risk Factors and Biological Behavior
Feline oral squamous cell carcinoma (FOSCC) is a promising and unique model for Human head and neck squamous cell carcinoma (HNSCC) [17]. Head and neck squamous cell carcinomas (HNSCC) are the most common oral neoplasia and the sixth most common cancer worldwide, counting for 890,000 new cases and 450,000 human deaths in 2018 [45].
In HNSCC, papillomavirus is considered an important risk factor as well as tobacco smoke and alcohol consumption [45]. Cats living in households with smokers are considered at increased risk of developing FOSCC compared to non-smoking households [46[46][47],47], possibly due to the deposition of chemicals from the tobacco smoke on the coat in conjunction with feline grooming habits. Cats with a high intake of canned food in their diet and cats wearing flea collars have also been reported to be more at risk of developing SCC [46]. The relationship of papilloma virus and FOSCC is not well established. In one study, 90% of feline cutaneous SCC carried papillomavirus DNA [48]. In a recent study using next generation sequencing, the presence of feline papillomavirus in FOSCC was very low, only 1 in 20 [49]. In contrast to the situation in people, papillomaviruses are unlikely to be a risk factor for FOSCC, hence, FOSCC is likely a better model for the more aggressive HPV negative HNSCC [49].
FOSCC is a common cancer in old cats and the most common tumor affecting the oral cavity [50]. (Figure 1) FOSCC is a locally aggressive tumor with a low metastatic rate. The mucosa of the tongue, mandible and maxilla are the most common sites [51]. At presentation, metastatic rate is low, with around 14–18% having metastasized to the regional lymph nodes and around 12% to the lungs [51]. Most patients are likely to die due to the consequences of the primary tumor which impacts the ability to eat and drink [51,52][51][52]. Like FOSCC, HNSCC is a local aggressive disease with early invasion and destruction of the surrounding tissues, and metastases presenting only at a later stage [45]. Humans, like feline patients, often present at advanced stages as precancerous oral lesions are rare [45].
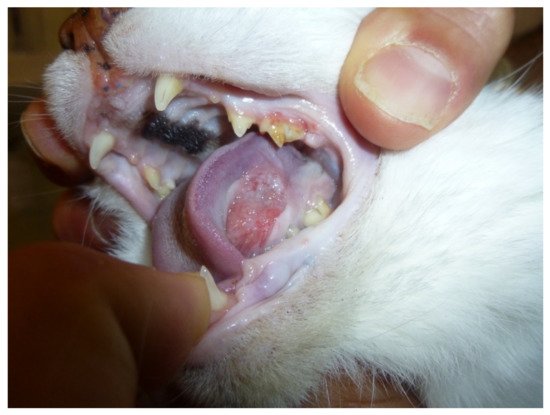
Figure 1. Sublingual oral squamous cell carcinoma in a 13-year-old female domestic shorthair cat.
3. Canine Oral Melanoma as a Translational Model of Mucosal Melanoma and Immunotherapy in People
Biological Behavior and Molecular Similarities
Canine oral melanoma might represent a unique model to study mucosal melanoma in people, and to assess the safety and efficacy of new immunotherapy drugs before human clinical trials.
Canine melanoma is a very common tumor in old dogs and the most common malignant tumor of the oral cavity [79][53] (Figure 2). The tumor originates from the neoplastic transformation of resident melanocytes of the oral cavity. Human mucosal melanoma is a rare cancer that affects mainly the oropharyngeal and nasal cavity [80][54]. The etiopathogenesis of human mucosal melanoma, in contrast to that of cutaneous melanoma, is largely unknown. The lack of exposure to UV-light rules this out as a causal factor for mucosal melanoma [81][55]. The risk factors and etiopathogenesis of oral melanoma in dogs are also unknown, but a genetic predisposition has been hypothesized due to the predisposition of some small breeds with heavily pigmented oral mucous membranes [82][56]. Mucosal melanomas in dogs are locally aggressive tumors with a high rate of metastasis especially to the loco-regional lymph nodes and lungs [79,83][53][57]. Contrary to people, canine cutaneous melanoma or melanocytoma are usually benign lesions often cured by a complete surgical excision [83][57]. In people, mucosal melanoma is much less common compared with dogs but shares similar aggressive biological behavior [44].The prognosis for mucosal melanoma in people is poor, with only around 20–30% of patients alive at five years [80,84][54][58]. Similarly, in dogs, advanced stage oral melanoma carries a very poor prognosis with survival ranging from two to five months [79,82,83][53][56][57]. Mucosal melanoma in dogs and people share a similar histopathological appearance and molecular/genetic background [44]. Oncogene mutations commonly found in cutaneous melanoma in humans, like BRAF and NRAS, are uncommon in mucosal melanoma in either humans or dogs, while activation of the ERK and AKT signaling pathways are common in both species [44]. Aberrant expression of the oncogene KIT and mutation of platelet derived growth factor receptor, PDGFRA, are more common in mucosal melanoma compared to cutaneous melanoma in people [85][59]. In canine oral melanoma, KIT mutation is uncommon and anti-KIT targeted therapy has resulted in only modest results [49,86][49][60]. However, PDGFRα/β expression was found in around 50% of oral canine melanoma and α and β co-expression was shown to correlate with a worse prognosis [87][61].


Figure 2. Mucosal melanoma of the oral cavity in a 9-year-old mixed breed dog.
References
- Ledford, H. Translational research: 4 ways to fix the clinical trial. Nature 2011, 477, 526–528.
- Cook, N.; Jodrell, D.I.; Tuveson, D.A. Predictive in vivo animal models and translation to clinical trials. Drug Discov. Today 2012, 17, 253–260.
- Mak, I.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114–118.
- Perel, P.; Roberts, I.; Sena, E.S.; Wheble, P.; Briscoe, C.; Sandercock, P.; Macleod, M.R.; Mignini, L.E.; Jayaram, P.; Khan, K.S. Comparison of treatment effects between animal experiments and clinical trials: Systematic review. BMJ 2007, 334, 197.
- Moreno, L.; Pearson, A.D.J. How Can Attrition Rates Be Reduced in Cancer Drug Discovery? Expert Opin. Drug Discov. 2013, 8, 363–368.
- Zhang, W. Mouse Models for Cancer Research. Chin. J. Cancer 2011, 30, 149–152.
- Cekanova, M.; Rathore, K. Animal models and therapeutic molecular targets of cancer: Utility and limitations. Dev. Ther. 2014, 8, 1911.
- Gawrylewski, A. The Trouble with Animal Models. Scientist 2007, 21, 45–51.
- Chesler, E.J.; Wilson, S.G.; Lariviere, W.R.; Rodriguez-Zas, S.L.; Mogil, J.S. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci. Biobehav. Rev. 2002, 26, 907–923.
- Khanna, C.; Lindblad-Toh, K.; Vail, D.; London, C.; Bergman, P.; Barber, L.; Breen, M.; Kitchell, B.; McNeil, E.; Modiano, J.F.; et al. The dog as a cancer model. Nat. Biotechnol. 2006, 24, 1065–1066.
- Paoloni, M.; Khanna, C. Translation of new cancer treatments from pet dogs to humans. Nat. Rev. Cancer 2008, 8, 147–156.
- Lindblad-Toh, K.; Members, B.S.P.; Wade, C.M.; Mikkelsen, T.S.; Karlsson, E.K.; Jaffe, D.B.; Kamal, M.; Clamp, M.; Chang, J.L.; Kulbokas, E.J.; et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nat. Cell Biol. 2005, 438, 803–819.
- Di Cerbo, A.; Palmieri, B.; de Vico, G.; Iannitti, T. Onco-Epidemiology of Domestic Animals and Targeted Therapeutic Attempts: Perspectives on Human Oncology. J. Cancer Res. Clin. Oncol. 2014, 140, 1807–1814.
- Riccardo, F.; Aurisicchio, L.; Impellizeri, J.A.; Cavallo, F. The importance of comparative oncology in translational medicine. Cancer Immunol. Immunother. 2015, 64, 137–148.
- Vascellari, M.; Baioni, E.; Ru, G.; Carminato, A.; Mutinelli, F. Animal tumour registry of two provinces in northern Italy: Incidence of spontaneous tumours in dogs and cats. BMC Vet. Res. 2009, 5, 39.
- Egenvall, A.; Bonnett, B.N.; Hedhammar, A.; Olson, P. Mortality in over 350,000 Insured Swedish Dogs from 1995–2000: II. Breed-Specific Age and Survival Patterns and Relative Risk for Causes of Death. Acta Vet. Scand. 2005, 46, 121–136.
- Wypij, J.M. A Naturally Occurring Feline Model of Head and Neck Squamous Cell Carcinoma. Pathol. Res. Int. 2013, 2013, 502197.
- Cmrečak, F.; Andrašek, I.; Gregov, V.; Beketić-Orešković, L. Obesity and cancer. Libr. Oncol. 2020, 48, 89–102.
- Sapowicz, S.A.; Linder, D.E.; Freeman, L.M. Body Condition Scores and Evaluation of Feeding Habits of Dogs and Cats at a Low Cost Veterinary Clinic and a General Practice. Sci. World J. 2016, 2016, 1901679.
- Linder, D.E.; Santiago, S.; Halbreich, E.D. Is There a Correlation Between Dog Obesity and Human Obesity? Preliminary Findings of Overweight Status Among Dog Owners and Their Dogs. Front. Vet. Sci. 2021, 8, 654617.
- Alvarez, C.E. Naturally Occurring Cancers in Dogs: Insights for Translational Genetics and Medicine. ILAR J. 2014, 55, 16–45.
- Rowell, J.L.; McCarthy, D.O.; Alvarez, C.E. Dog models of naturally occurring cancer. Trends Mol. Med. 2011, 17, 380–388.
- Vail, D.M.; Thamm, D.H. Spontaneously occurring tumors in companion animals as models for drug development. In Anticancer Drug Development Guide; Humana Press: Totowa, NJ, USA, 2004; pp. 259–284.
- Valerius, K.D.; Ogilvie, G.K.; Mallinckrodt, C.H.; Getzy, D.M. Doxorubicin alone or in combination with asparaginase, followed by cyclophosphamide, vincristine, and prednisone for treatment of multicentric lymphoma in dogs: 121 cases (1987–1995). J. Am. Vet. Med. Assoc. 1997, 210, 512–516.
- Marech, I.; Patruno, R.; Zizzo, N.; Gadaleta, C.; Introna, M.; Zito, A.F.; Gadaleta, C.D.; Ranieri, G. Masitinib (AB1010), from Canine Tumor Model to Human Clinical Development: Where We Are? Crit. Rev. Oncol. 2014, 91, 98–111.
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089.
- Andreeva, N.V.; Gabbasova, R.R.; Grivennikov, S.I. Microbiome in cancer progression and therapy. Curr. Opin. Microbiol. 2020, 56, 118–126.
- O’Neill, K.; Guth, A.; Biller, B.; Elmslie, R.; Dow, S. Changes in Regulatory T Cells in Dogs with Cancer and Associations with Tumor Type. J. Vet. Intern. Med. 2009, 23, 875–881.
- Biller, B.; Guth, A.; Burton, J.; Dow, S. Decreased Ratio of CD8+ T Cells to Regulatory T Cells Associated with Decreased Survival in Dogs with Osteosarcoma. J. Vet. Intern. Med. 2010, 24, 1118–1123.
- Garden, O.; Pinheiro, D.; Cunningham, F. All creatures great and small: Regulatory T cells in mice, humans, dogs and other domestic animal species. Int. Immunopharmacol. 2011, 11, 576–588.
- Maekawa, N.; Konnai, S.; Okagawa, T.; Nishimori, A.; Ikebuchi, R.; Izumi, Y.; Takagi, S.; Kagawa, Y.; Nakajima, C.; Suzuki, Y.; et al. Immunohistochemical Analysis of PD-L1 Expression in Canine Malignant Cancers and PD-1 Expression on Lymphocytes in Canine Oral Melanoma. PLoS ONE 2016, 11, e0157176.
- Stevenson, V.B.; Perry, S.N.; Todd, M.; Huckle, W.R.; LeRoith, T. PD-1, PD-L1, and PD-L2 Gene Expression and Tumor Infiltrating Lymphocytes in Canine Melanoma. Vet. Pathol. 2021, 58, 692–698.
- Hartley, G.; Elmslie, R.; Dow, S.; Guth, A. Checkpoint molecule expression by B and T cell lymphomas in dogs. Vet. Comp. Oncol. 2018, 16, 352–360.
- Wang, Y.J.; Pietrzak, K.; Kocikowski, M.; Argyle, D.; Hupp, T.; Parys, M. Investigation of Tumor Intrinsic PD1/PD-L1 in Canine Urothelial Carcinomas as a Spontaneous Translational Model for Human Invasive Bladder Cancer. Cancer Res. 2019, 79, 3972.
- Pinard, C.J.; Stegelmeier, A.A.; Bridle, B.W.; Mutsaers, A.J.; Wood, R.D.; Wood, G.A.; Woods, J.P.; Hocker, S.E. Evaluation of lymphocyte-specific PD-1 receptor expression and cytokines in blood and urine in canine urothelial carcinoma patients. Vet. Comp. Oncol. 2021, 1–10.
- Bergman, P.J.; McKnight, J.; Novosad, A.; Charney, S.; Farrelly, J.; Craft, D.; Wulderk, M.; Jeffers, Y.; Sadelain, M.; Hohenhaus, A.E.; et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: A phase I trial. Clin. Cancer Res. 2003, 9, 1284–1290.
- Liao, J.C.F.; Gregor, P.; Wolchok, J.D.; Orlandi, F.; Craft, D.; Leung, C.; Houghton, A.N.; Bergman, P.J. Vaccination with human tyrosinase DNA induces antibody responses in dogs with advanced melanoma. Cancer Immun. 2006, 6, 8.
- Mason, N.J.; Gnanandarajah, J.S.; Engiles, J.B.; Gray, F.; Laughlin, D.S.; Gaurnier-Hausser, A.; Wallecha, A.; Huebner, M.; Paterson, Y. Immunotherapy with a HER2-Targeting Listeria Induces HER2-Specific Immunity and Demonstrates Potential Therapeutic Effects in a Phase I Trial in Canine Osteosarcoma. Clin. Cancer Res. 2016, 22, 4380–4390.
- Nguyen, S.M.; Thamm, D.; Vail, D.M.; London, C.A. Response evaluation criteria for solid tumours in dogs (v1.0): A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 2015, 13, 176–183.
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary Cooperative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) following investigational therapy in dogs and cats. Vet. Comp. Oncol. 2021, 19, 311–352.
- Reform Regulations to Make Pet Clinical Trials Easier. Nature 2016, 540, 169.
- Kendall, L.V.; Petervary, N.; Bergdall, V.K.; Page, R.L.; Baneux, P.J.R. Institutional animal care and use committee review of clinical studies. J. Am. Vet. Med. Assoc. 2018, 253, 980–984.
- Hampshire, V.A. Regulatory issues surrounding the use of companion animals in clinical investigations, trials, and studies. ILAR J. 2003, 44, 191–196.
- Simpson, R.M.; Bastian, B.; Michael, H.; Webster, J.D.; Prasad, M.L.; Conway, C.M.; Prieto, V.M.; Gary, J.M.; Goldschmidt, M.; Esplin, D.G.; et al. Sporadic naturally occurring melanoma in dogs as a preclinical model for human melanoma. Pigment Cell Melanoma Res. 2014, 27, 37–47.
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92.
- Bertone, E.R.; Snyder, L.A.; Moore, A.S. Environmental and Lifestyle Risk Factors for Oral Squamous Cell Carcinoma in Domestic Cats. J. Vet. Intern. Med. 2003, 17, 557–562.
- Snyder, L.A.; Bertone, E.R.; Jakowski, R.M.; Dooner, M.S.; Jennings-Ritchie, J.; Moore, A.S. P53 Expression and Environmental Tobacco Smoke Exposure in Feline Oral Squamous Cell Carcinoma. Vet. Pathol. 2004, 41, 209–214.
- Munday, J.S.; Kiupel, M.; French, A.F.; Howe, L. Amplification of papillomaviral DNA sequences from a high proportion of feline cutaneousin situand invasive squamous cell carcinomas using a nested polymerase chain reaction. Vet. Dermatol. 2008, 19, 259–263.
- Chu, S.; Wylie, T.N.; Wylie, K.M.; Johnson, G.C.; Skidmore, Z.L.; Fleer, M.; Griffith, O.L.; Bryan, J.N. A virome sequencing approach to feline oral squamous cell carcinoma to evaluate viral causative factors. Vet. Microbiol. 2020, 240, 108491.
- Cotter, S.M. Oral Pharyngeal Neoplasms in the Cat. J. Am. Anim. Hosp. Assoc. 1981, 17, 917–920.
- Marretta, J.J.; Garrett, L.D.; Marretta, S.M. Feline Oral Squamous Cell Carcinoma: An Overview. Vet. Med. 2007, 102, 392–406.
- Giuliano, A.; Swift, R.; Arthurs, C.; Marote, G.; Abramo, F.; McKay, J.; Thomson, C.; Beltran, M.; Millar, M.; Priestnall, S.; et al. Quantitative Expression and Co-Localization of Wnt Signalling Related Proteins in Feline Squamous Cell Carcinoma. PLoS ONE 2016, 11, e0161103.
- Modiano, J.F.; Ritt, M.G.; Wojcieszyn, J. The Molecular Basis of Canine Melanoma: Pathogenesis and Trends in Diagnosis and Therapy. J. Vet. Intern. Med. 1999, 13, 163–174.
- Temam, S.; Mamelle, G.; Marandas, P.; Wibault, P.; Avril, M.-F.; Janot, F.; Julieron, M.; Schwaab, G.; Luboinski, B. Postoperative radiotherapy for primary mucosal melanoma of the head and neck. Cancer 2005, 103, 313–319.
- Tacastacas, J.D.; Bray, J.; Cohen, Y.K.; Arbesman, J.; Kim, J.; Koon, H.B.; Honda, K.; Cooper, K.D.; Gerstenblith, M.R. Update on Primary Mucosal Melanoma. J. Am. Acad. Dermatol. 2014, 71, 366–375.
- Ramos-Vara, J.A.; Beissenherz, M.E.; Miller, M.A.; Johnson, G.C.; Pace, L.W.; Fard, A.; Kottler, S.J. Retrospective Study of 338 Canine Oral Melanomas with Clinical, Histologic, and Immunohistochemical Review of 129 Cases. Vet. Pathol. 2000, 37, 597–608.
- Bostock, D.E. Prognosis after Surgical Excision of Canine Melanomas. Vet. Pathol. 1979, 16, 32–40.
- Jethanamest, D.; Vila, P.; Sikora, A.G.; Morris, L.G.T. Predictors of Survival in Mucosal Melanoma of the Head and Neck. Ann. Surg. Oncol. 2011, 18, 2748–2756.
- Curtin, J.A.; Busam, K.; Pinkel, D.; Bastian, B.C. Somatic Activation of KIT in Distinct Subtypes of Melanoma. J. Clin. Oncol. 2006, 24, 4340–4346.
- Giuliano, A.; Dobson, J. Prospective clinical trial of masitinib mesylate treatment for advanced stage III and IV canine malignant melanoma. J. Small Anim. Pract. 2020, 61, 190–194.
- Iussich, S.; Maniscalco, L.; Di Sciuva, A.; Iotti, B.; Morello, E.; Martano, M.; Gattino, F.; Buracco, P.; De Maria, R. PDGFRs expression in dogs affected by malignant oral melanomas: Correlation with prognosis. Vet. Comp. Oncol. 2017, 15, 462–469.
More
