The cholecystokinin-2 receptor (CCK2R) is a promising target for theranostic use in nuclear medicine, and has been in the focus of the radiopharmaceutical development over the last twenty years. The expression of this receptor at high incidence and density has been proven mainly for medullary thyroid carcinoma (MTC) and small cell lung cancer (SCLC). Furthermore, CCK2R expression has been confirmed for gastrointestinal stromal tumors, astrocytomas and stromal ovarian cancers. In addition, CCK2R targeting might be of additive value for gastroenteropancreatic and bronchopulmonary neuroendocrine tumors, especially insulinomas, vipomas, as well as bronchial and ileal carcinoids. Most of the clinical experience with CCK2R targeting radiopharmaceuticals has been gained for patients with advanced MTC. Therefore, the diagnostic and therapeutic potential of CCK2R targeting is documented mainly for this patient group.
- cholecystokinin-2 receptor
- tumor targeting
- molecular imaging
- targeted radiotherapy
1. Introduction
The development of radiolabeled CCK2R targeting peptide analogs was initiated in the late 1990s. Several pioneers laid the foundation for this new diagnostic and therapeutic approach. Strong CCK2R expression, especially in MTC and its metastases, was demonstrated by the group of Prof. Jean Claude Reubi [4][1]. First scintigraphic visualization of tumor lesions in a patient with metastasised MTC using 131I-labeled gastrin was undertaken by Thomas Behr in 1998 [5][2]. In this team, Martin Béhé developed the first 111In-labeled gastrin derivatives with selective affinity for CCK2R [5,6][2][3]. The research group around Marion de Jong worked on non-sulphated cholecystokinin analogs. By increasing specificity for CCK2R over CCK1R, reduced uptake in normal tissue expressing CCK1R was achieved [7][4]. Soon thereafter, additional research groups across Europe directed their attention to the preclinical development of peptide-based CCK2R targeting probes.
Since the very beginning, the difficulties in the development of clinically useful radiolabeled CCK2R targeting peptide analogs became clear. These were related either to high kidney uptake, leading to nephrotoxicity during therapeutic application, or low enzymatic stability, limiting the tumor targeting properties [8,9][5][6]. The clinical comparison of three different 111In- and 99m Tc-labeled derivatives of human minigastrin (MG) and cholecystokinin-8 demonstrated the need for further radiopharmaceutical development to enable CCK2R-based peptide receptor radionuclide therapy (PRRT) [10][7]. A small, but well networked radiopharmaceutical community accelerated the radiopharmaceutical development. Within the COST (European Cooperation in Science and Technology) Action BM0607 on Targeted Radionuclide Therapy, twelve different CCK2R targeting peptide analogs were preclinically evaluated, with the major aim of finding a promising new candidate for PRRT [11,12,13][8][9][10]. These joint efforts triggered further clinical studies and gave new directions to the ongoing preclinical research.
2. Tumor Imaging with Radiolabeled Gastrin Analogs
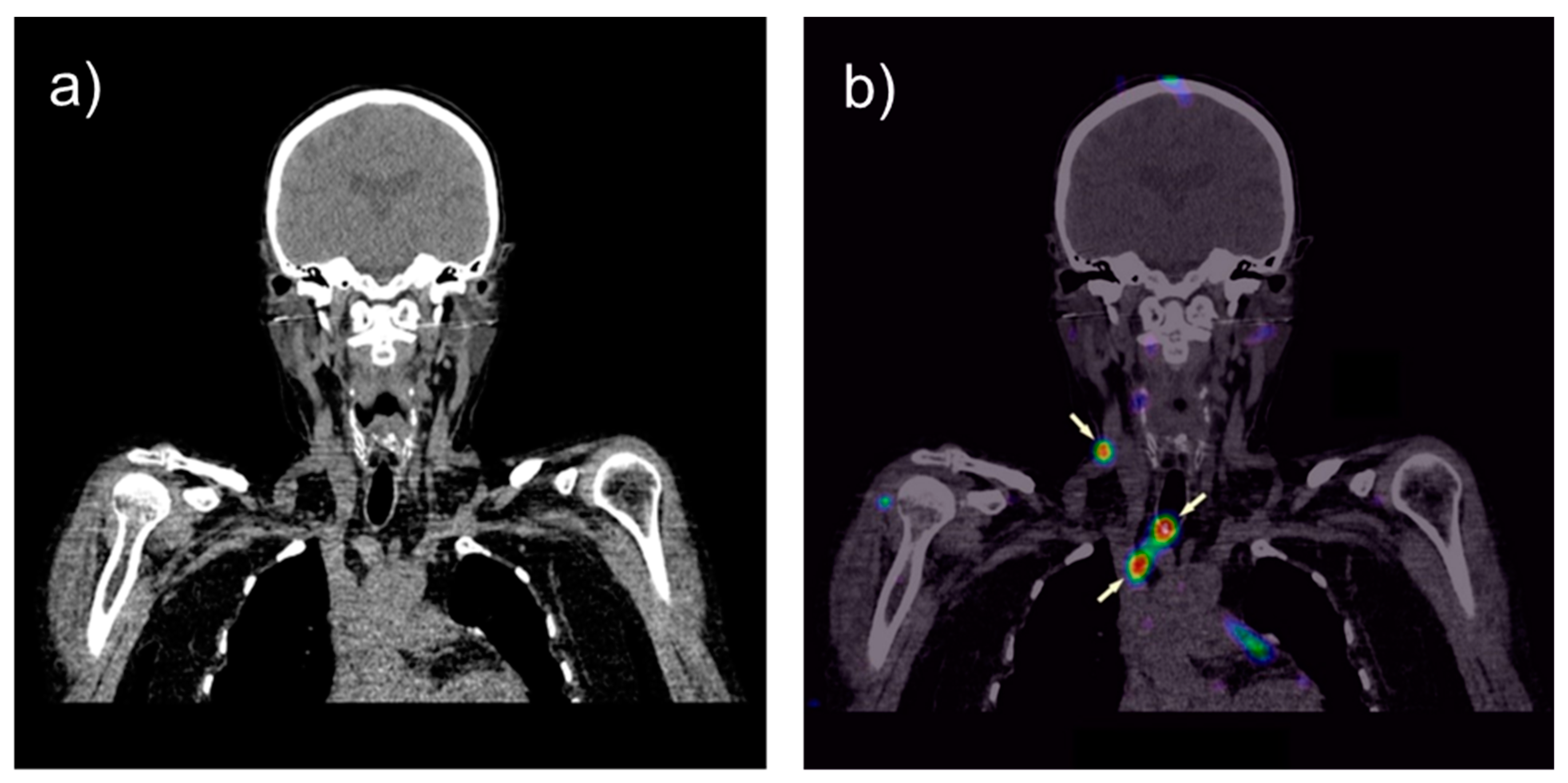
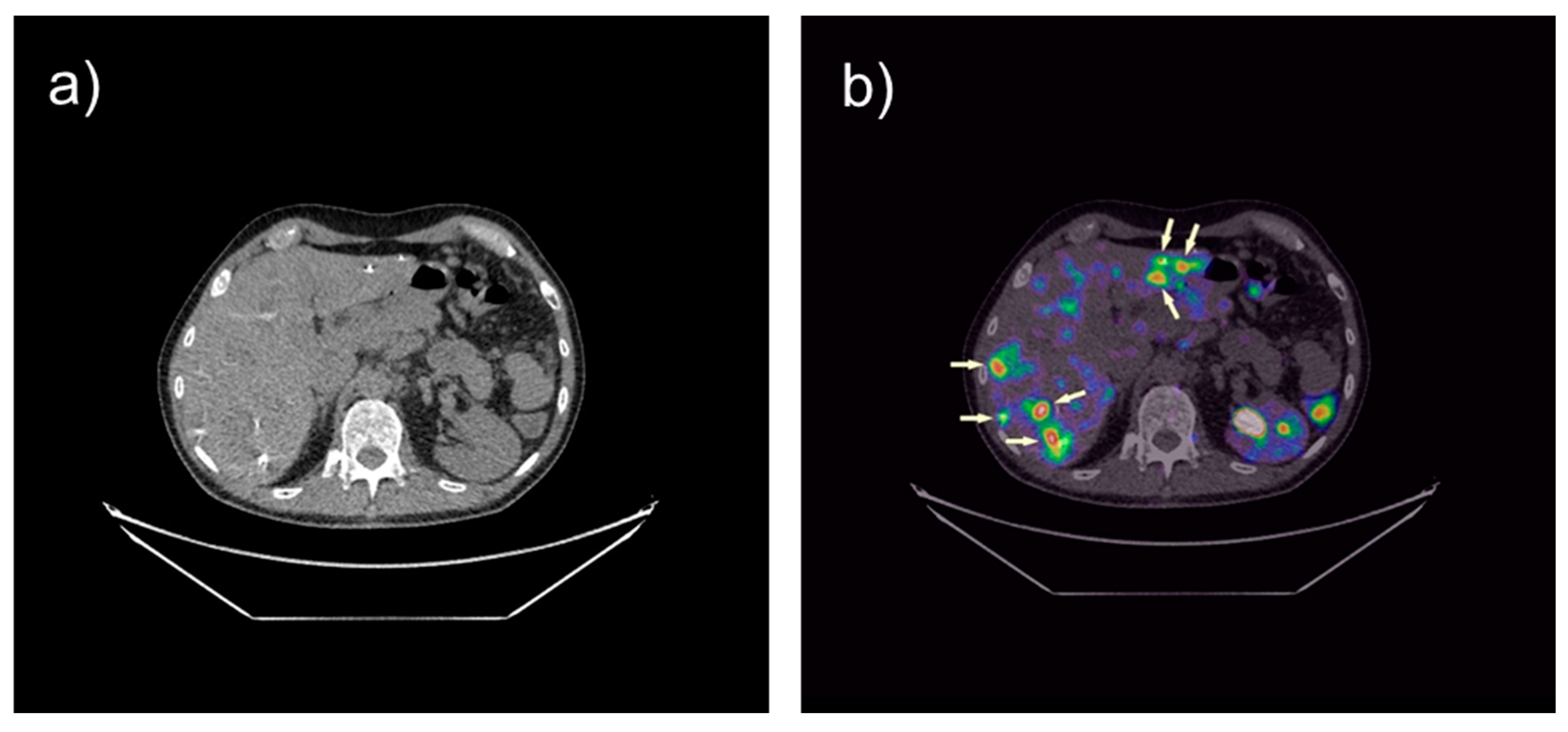
3. Tumor Therapy with Radiolabeled Gastrin Analogs
CCK2R is also an interesting target for PRRT. Among tumors expressing CCK2R, MTC is of special interest because of its high receptor incidence in more than 90% of MTC [1][31] and, on the other hand, the limited therapy options in patients with advanced stages of MTC. Standard therapy of MTC consists in total thyroidectomy and compartment-oriented lymph node dissection, which is the only chance to achieve cure. Patients with postoperative normalisation of the tumor marker calcitonin can be considered “biochemically cured” and have a favorable prognosis [55][13]. However, in roughly 10% of all patients, distant metastases are present already at the time of diagnosis, and are discovered in further 19–38% of patients during follow-up [55][13]. Furthermore, initial postoperative biochemical cure is very unlikely in patients with >10 lymph node metastases, and the chances to achieve a long-term biochemical cure are only realistic in patients with very low numbers of positive nodes [58,70][16][32]. Until several years ago, when the multikinase inhibitors cabozantinib and vandetanib were introduced as first-line systemic therapy for MTC, systemic treatment options for MTC patients were very limited, since chemotherapy has historically yielded poor results and MTC lacks uptake of radioiodine [55,58][13][16]. For tumors harbouring “rearranged during transfection” (RET) mutations, the situation recently improved further with the approval of selective inhibitors of the RET-oncogene, showing higher response rates combined with less side effects [71][33]. However, the systemic therapies currently approved for MTC have not been shown to improve overall survival yet, and evidence-based guidance on when to start these therapies in patients is still lacking [55][13]. Therefore, the need for further, effective systemic therapies remains. MTC was chosen for the first and to date the only published treatment (phase I) study with the CCK2R agonist [90Y]Y-DTPA-MG0 [8][5]. Eight patients with advanced and rapidly progressing metastatic MTC received up to 1.85 GBq/m2 body surface, resulting in partial remission (n = 2), stable disease (n = 4) and progressive disease (n = 2) within a follow-up period of 12-36 months. Unfortunately, high hematologic and renal toxicity prevented further trials with this compound. In the course of the following years, further development resulted in MG analogs with improved biodistribution. One of these, [177Lu]Lu-PP-F11N, was chosen for a clinical phase 0 trial as a proof of principle study (ClinicalTrials.gov (accessed on 22 September 2021): NCT02088645) [20][34]. This compound showed a promising biodistribution with specific uptake in MTC. Dosimetry after infusion of 1 GBq of [177Lu]Lu-PP-F11N revealed radiation doses to MTC lesions sufficient for therapy, combined with low radiation doses absorbed in kidneys and bone marrow. The highest radiation doses were seen in the stomach, which would most likely be the dose-limiting organ. The administration of Gelofusine revealed no significant effect on the already low dose absorbed by the kidneys. The acute toxicity of [177Lu]Lu-PP-F11N was low with self-limiting adverse events not higher than grade 1, according to Common Terminology Criteria for Adverse Events version 4.03 (CTCAE). Based on the dosimetric calculations, it was stated that fractionated therapy with 50 GBq [177Lu]Lu-PP-F11N should be possible without surpassing maximum tolerated doses of risk organs. In consequence, a phase 1 dose escalation study was initiated (ClinicalTrials.gov (accessed on 22 September 2021): NCT02088645). Within this trial, the first planned escalation cohort (n = 3) received 3 × 6 GBq without dose limiting toxicity or other toxicity higher than grade 2, according to CTCAE [72][35]. In all patients, at least a temporary reduction of tumor markers occurred after therapy. In Figure 3, an exemplary SPECT/CT image of a patient with hepatic MTC metastases is given, showing specific uptake of [177Lu]Lu-PP-F11N in the liver lesions together with physiological uptake in the stomach. Therefore, the study continued with the next dose escalation step (4 × 8 GBq).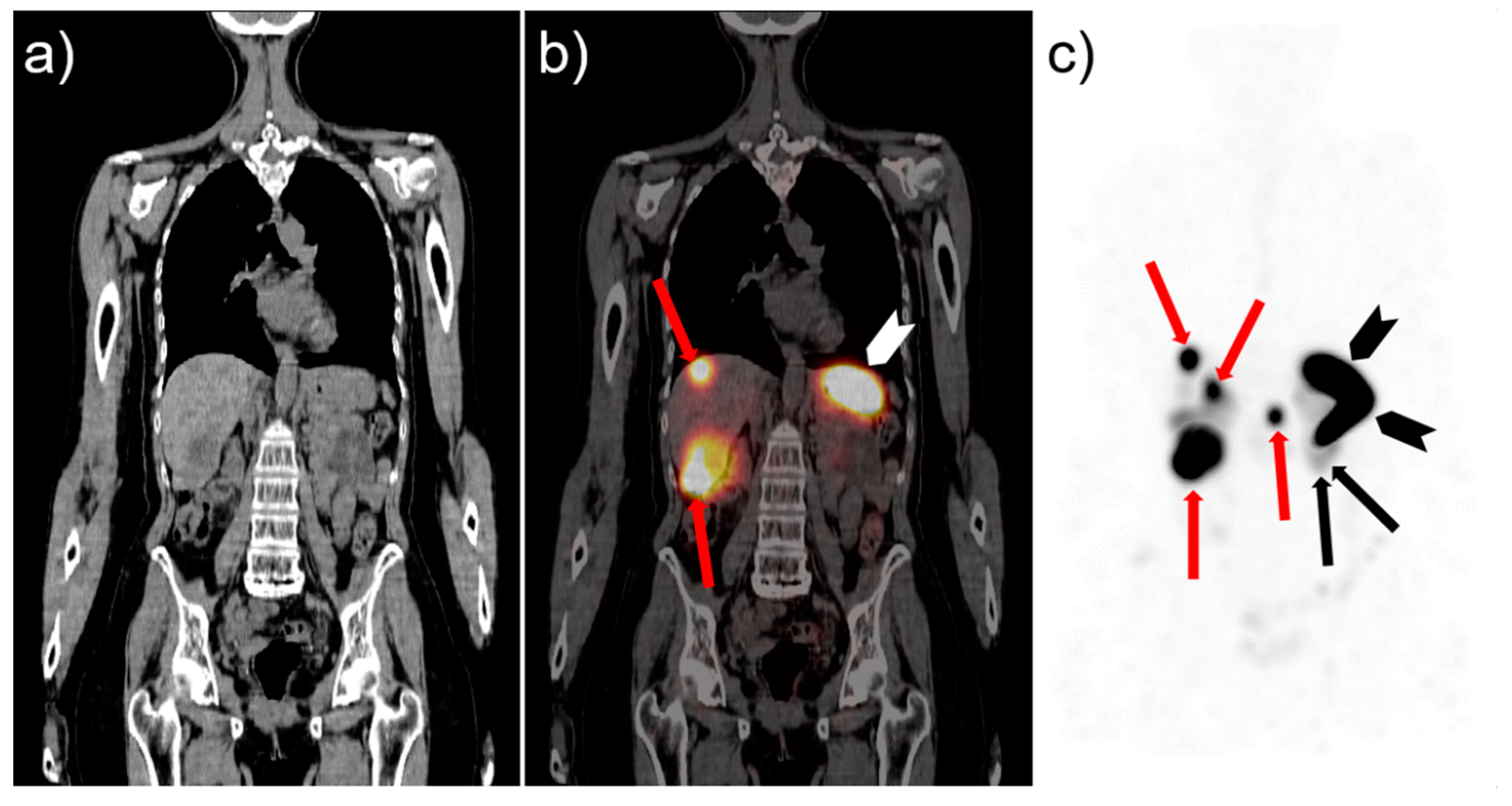
References
- Reubi, J.C.; Waser, B. Unexpected high incidence of cholecystokinin-B/gastrin receptors in human medullary thyroid carcinomas. Int. J. Cancer 1996, 67, 644–647.
- Behr, T.M.; Jenner, N.; Radetzky, S.; Behe, M.; Gratz, S.; Yücekent, S.; Raue, F.; Becker, W. Targeting of cholecystokinin-B/gastrin receptors in vivo: Preclinical and initial clinical evaluation of the diagnostic and therapeutic potential of radiolabelled gastrin. Eur. J. Nucl. Med. 1998, 25, 424–430.
- Behr, T.M.; Jenner, N.; Behe, M.; Angerstein, C.; Gratz, S.; Raue, F.; Becker, W. Radiolabeled peptides for targeting cholecystokinin-B/gastrin receptor-expressing tumors. J. Nucl. Med. 1999, 40, 1029–1044.
- de Jong, M.; Bakker, W.H.; Bernard, B.F.; Valkema, R.; Kwekkeboom, D.J.; Reubi, J.C.; Srinivasan, A.; Schmidt, M.; Krenning, E.P. Preclinical and initial clinical evaluation of 111In-labeled nonsulfated CCK8 analog: A peptide for CCK-B receptor-targeted scintigraphy and radionuclide therapy. J. Nucl. Med. 1999, 40, 2081–2087.
- Behe, M.; Behr, T.M. Cholecystokinin-B (CCK-B)/gastrin receptor targeting peptides for staging andtherapy of medullary thyroidcancer and other CCK-B receptor expressing malignancies. Biopolymers 2002, 66, 399–418.
- Breeman, W.A.; Froberg, A.C.; de Blois, E.; van Gameren, A.; Melis, M.; de Jong, M.; Maina, T.; Nock, B.A.; Erion, J.L.; Macke, H.R.; et al. Optimised labeling, preclinical and initial clinical aspects of CCK-2 receptor-targeting with 3 radiolabeled peptides. Nucl. Med. Biol. 2008, 35, 839–849.
- Fröberg, A.C.; de Jong, M.; Nock, B.A.; Breeman, W.A.; Erion, J.L.; Maina, T.; Verdijsseldonck, M.; de Herder, W.W.; van der Lugt, A.; Kooij, P.P.; et al. Comparison of three radiolabelled peptide analogues for CCK-2 receptor scintigraphy in medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1265–1272.
- Aloj, L.; Aurilio, M.; Rinaldi, V.; D’Ambrosio, L.; Tesauro, D.; Peitl, P.K.; Maina, T.; Mansi, R.; von Guggenberg, E.; Joosten, L.; et al. Comparison of the binding and internalization properties of 12 DOTA-coupled and 111In-labelled CCK2/gastrin receptor binding peptides: A collaborative project under COST Action BM0607. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1417–1425.
- Laverman, P.; Joosten, L.; Eek, A.; Roosenburg, S.; Peitl, P.K.; Maina, T.; Macke, H.; Aloj, L.; von Guggenberg, E.; Sosabowski, J.K.; et al. Comparative biodistribution of 12 111In-labelled gastrin/CCK2 receptor-targeting peptides. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1410–1416.
- Ocak, M.; Helbok, A.; Rangger, C.; Peitl, P.K.; Nock, B.A.; Morelli, G.; Eek, A.; Sosabowski, J.K.; Breeman, W.A.; Reubi, J.C.; et al. Comparison of biological stability and metabolism of CCK2 receptor targeting peptides, a collaborative project under COST BM0607. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1426–1435.
- Costante, G.; Meringolo, D. Calcitonin as a biomarker of C cell disease: Recent achievements and current challenges. Endocrine 2020, 67, 273–280.
- Vardarli, I.; Weber, M.; Weidemann, F.; Führer, D.; Herrmann, K.; Görges, R. Diagnostic accuracy of routine calcitonin measurement for the detection of medullary thyroid carcinoma in the management of patients with nodular thyroid disease: A meta-analysis. Endocr. Connect. 2021, 10, 358–370.
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann. Oncol. 2019, 30, 1856–1883.
- Network, N.C.C. Thyroid Carcinoman (Version 2). Available online: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf (accessed on 22 September 2021).
- Treglia, G.; Aktolun, C.; Chiti, A.; Frangos, S.; Giovanella, L.; Hoffmann, M.; Iakovou, I.; Mihailovic, J.; Krause, B.J.; Langsteger, W.; et al. The 2015 Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma: The “evidence-based” refusal to endorse them by EANM due to the “not evidence-based” marginalization of the role of Nuclear Medicine. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1486–1490.
- Wells, S.A., Jr.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised american thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610.
- Giovanella, L.; Treglia, G.; Iakovou, I.; Mihailovic, J.; Verburg, F.A.; Luster, M. EANM practice guideline for PET/CT imaging in medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 61–77.
- Lee, S.W.; Shim, S.R.; Jeong, S.Y.; Kim, S.J. Comparison of 5 different pet radiopharmaceuticals for the detection of recurrent medullary thyroid carcinoma: A network meta-analysis. Clin. Nucl. Med. 2020, 45, 341–348.
- Erba, P.A.; Maecke, H.; Mikolajczak, R.; Decristoforo, C.; Zaletel, K.; Maina-Nock, T.; Peitl, P.K.; Garnuszek, P.; Fröberg, A.; Goebel, G.; et al. A novel CCK2/gastrin receptor-localizing radiolabeled peptide probe for personalized diagnosis and therapy of patients with progressive or metastatic medullary thyroid carcinoma: A multicenter phase I GRAN-T-MTC study. Pol. Arch. Intern. Med. 2018, 128, 791–795.
- Kolenc Peitl, P.; Tamma, M.; Kroselj, M.; Braun, F.; Waser, B.; Reubi, J.C.; Sollner Dolenc, M.; Maecke, H.R.; Mansi, R. Stereochemistry of amino acid spacers determines the pharmacokinetics of 111In−DOTA−minigastrin analogues for targeting the CCK2/gastrin receptorr. Bioconjug. Chem. 2015, 26, 1113–1119.
- Schwarzenböck, S.M.; Garibotto, V. Highlights of the 32th annual congress of the EANM, Barcelona 2019: The nucleolympic games of nuclear medicine-a global competition for excellence. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1808–1819.
- Hubalewska-Dydejczyk, A.; Mikolajczak, R.; Decristoforo, C.; Kolenc-Peitl, P.; Erba, P.; Zaletel, K.; Maecke, H.; Maina, T.; Konijnenberg, M.; Garnuszek, P. Phase I clinical trial using a novel CCK 2 receptor-localizing radiolabelled peptide probe for personalized diagnosis and therapy of patients with progressive or metastatic medullary thyroid carcinoma-final results. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, S339.
- Klingler, M.; Hörmann, A.A.; Guggenberg, E.V. Cholecystokinin-2 receptor targeting with radiolabeled peptides: Current status and future directions. Curr. Med. Chem. 2020, 27, 7112–7132.
- Kunikowska, J.; Ziemnicka, K.; Pawlak, D.; Ruchala, M.; Kolasa, A.; Janicka-Jedynska, M.; Wozniak, A.; Mikolajczak, R.; Krolicki, L. Medullary thyroid carcinoma—PET/CT imaging with 68Ga-labelled gastrin and somatostatin analogues. Endokrynol. Pol. 2016, 67, 68–71.
- Uprimny, C.; von Guggenberg, E.; Svirydenka, A.; Mikolajczak, R.; Hubalewska-Dydejczyk, A.; Virgolini, I.J. Comparison of PET/CT imaging with FDOPA and cholecystokinin-2 receptor targeting Ga-DOTA-MGS5 in a patient with advanced medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 935–936.
- Reubi, J.C.; Waser, B. Concomitant expression of several peptide receptors in neuroendocrine tumours: Molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 781–793.
- Elisei, R.; Tacito, A.; Ramone, T.; Ciampi, R.; Bottici, V.; Cappagli, V.; Viola, D.; Matrone, A.; Lorusso, L.; Valerio, L.; et al. Twenty-five years experience on ret genetic screening on hereditary mtc: An update on the prevalence of germline ret mutations. Genes 2019, 10, 698.
- Raue, F.; Frank-Raue, K. Update on multiple endocrine neoplasia type 2: Focus on medullary thyroid carcinoma. J. Endocr. Soc. 2018, 2, 933–943.
- Salvatore, D.; Santoro, M.; Schlumberger, M. The importance of the RET gene in thyroid cancer and therapeutic implications. Nat. Rev. Endocrinol. 2021, 17, 296–306.
- Kushchayev, S.V.; Kushchayeva, Y.S.; Tella, S.H.; Glushko, T.; Pacak, K.; Teytelboym, O.M. Medullary thyroid carcinoma: An update on imaging. J. Thyroid Res. 2019, 2019, 1893047.
- Reubi, J.C.; Schaer, J.C.; Waser, B. Cholecystokinin(CCK)-A and CCK-B/gastrin receptors in human tumors. Cancer Res. 1997, 57, 1377–1386.
- Mathiesen, J.S.; Kroustrup, J.P.; Vestergaard, P.; Stochholm, K.; Poulsen, P.L.; Rasmussen, A.K.; Feldt-Rasmussen, U.; Schytte, S.; Londero, S.C.; Pedersen, H.B.; et al. Survival and long-term biochemical cure in medullary thyroid carcinoma in denmark 1997–2014: A nationwide study. Thyroid 2019, 29, 368–377.
- Wirth, L.J.; Sherman, E.; Robinson, B.; Solomon, B.; Kang, H.; Lorch, J.; Worden, F.; Brose, M.; Patel, J.; Leboulleux, S.; et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N. Engl. J. Med. 2020, 383, 825–835.
- Rottenburger, C.; Nicolas, G.P.; McDougall, L.; Kaul, F.; Cachovan, M.; Vija, A.H.; Schibli, R.; Geistlich, S.; Schumann, A.; Rau, T.; et al. Cholecystokinin 2 receptor agonist 177Lu-PP-F11N for radionuclide therapy of medullary thyroid carcinoma: Results of the lumed phase 0a study. J. Nucl. Med. 2020, 61, 520–526.
- Rottenburger, C.; Nicolas, G.; McDougall, L.; Fürstner, M.; Hentschel, M.; Kaul, F.; Christ, E.; Cachovan, M.; Vija, H.; Schibli, R. The CCK-2 receptor agonist Lu-177-PP-F11N for PRRT of medullary thyroid cancer–Recent results of the phase 1 “LUMED” Study. Nuklearmedizin 2021, 60, L15.
- Parghane, R.V.; Naik, C.; Talole, S.; Desmukh, A.; Chaukar, D.; Banerjee, S.; Basu, S. Clinical utility of 177Lu-DOTATATE PRRT in somatostatin receptor-positive metastatic medullary carcinoma of thyroid patients with assessment of efficacy, survival analysis, prognostic variables, and toxicity. Head Neck 2020, 42, 401–416.
- Salaun, P.Y.; Campion, L.; Bournaud, C.; Faivre-Chauvet, A.; Vuillez, J.P.; Taieb, D.; Ansquer, C.; Rousseau, C.; Borson-Chazot, F.; Bardet, S.; et al. Phase II trial of anticarcinoembryonic antigen pretargeted radioimmunotherapy in progressive metastatic medullary thyroid carcinoma: Biomarker response and survival improvement. J. Nucl. Med. 2012, 53, 1185–1192.
