Research has indicated that zinc plays a consequential mechanistic role in the protection against oxidative stress as zinc is required for the proper functioning of the antioxidant system, the suppression of inflammatory mediators, and the modulation of zinc transporters. Recently, the mechanisms surrounding ZnT8, ZIP7, and metallothionein have shown to be of particular pathogenic importance and are considered as potential therapeutic targets in disease management. The literature has shown that zinc dysregulation is associated with diabetes and may be considered as a leading contributor to the deleterious vascular alterations exhibited by the disease. Although further investigation is required, studies have indicated the favorable use of zinc supplementation in the protection against and prevention of oxidative stress and its consequences over the course of the condition.
1. Zinc Homeostasis
As Zn
2+ plays a diverse role in cellular processes including cell signaling, enzymatic activity, and gene transcription, homeostatic mechanisms are required to tightly control Zn
2+ absorption, distribution, intracellular availability, and excretion
[1][2][15,23]. The cation Zn
2+ cannot cross lipid bilayers and consequently physiological levels are maintained by three groups of proteins which regulate the inflow, outflow, and compartmentalization of Zn
2+: the ZnT and ZIP families of Zn
2+ transporters and the Zn
2+-sensitive metallothioneins (MTs)
[3][1][2][4][9,15,23,24]. The ZnT family (SLC30A) is a group of 10 (ZnT1-ZnT10) cation-diffusion facilitators that transport Zn
2+ ions towards the extracellular space or from the cytosol into organelles
[5][6][10,14]. The ZIP family (SLC39A), ZIP1-ZIP14, passes Zn
2+ into the cytoplasm from the extracellular space or from intracellular organelles
[5][7][6][10,11,14]. Zn
2+ binds with MTs until homeostatic conditions change such that Zn
2+ is required to be released and redistributed in the cells (e.g., in a state of oxidative stress, Zn
2+ is released from its complex with MT for antioxidant purposes)
[8][1][4,15].
Movement of the cation is facilitated within a bimodal framework of Zn
2+ signaling. Early zinc signaling (EZS) is independent of gene transcription and results in a rapid fluctuation of intracellular Zn
2+ levels via efflux from the organelles into the cytosol
[7][11]. Late zinc signaling (LZS) is slower than the response of EZS because it consists of transcriptional changes in genes and includes the use of storage proteins or transporters. Together, both systems regulate processes involved in metabolism, cell differentiation, proliferation, and growth
[7][9][10][6][1][11,12,13,14,15]. In the liver or muscle, evidence of Zn
2+ on cellular signaling is exemplified through the inhibition of protein tyrosine phosphatase 1B (PTP1B). This protein negatively regulates insulin-signaling pathways whereas Zn
2+ can extend the insulin signal through the insulin receptor via the inhibition of PTP1B
[5][7][10,11]. The human MT family consists of 12 operational MTs, with MT1 and MT2 as the major isoforms in the pancreas; these molecules bind Zn
2+ with high affinity, incorporating up to seven Zn
2+ ions per molecule
[11][3][12][13][8,9,25,26]. As MTs are equipped with reversible dissociation, they can act as Zn
2+ donors or acceptors
[11][3][12][13][8,9,25,26]. Downstream cell signaling is induced by releasing Zn
2+ either via the oxidation of the sulfur donors in the MT molecule or through the interaction of the MT with nitric oxide (NO)
[12][25].
Zinc Distribution in the β-Cell
To understand the molecular pathways involved in
Diabetes mellitus (DM)DM and disease processes like IR it is necessary to uncover the contribution that Zn
2+ and Zn
2+-transporter mechanisms make towards cell signaling. This will provide the comprehension necessary to delineate the important molecules or pathways that have the greatest therapeutic potential
[7][11].
Generally, mammalian cells cannot withstand high concentrations of Zn
2+ as it can quickly cause toxicity; however, a small amount is necessary to maintain homeostatic functioning, hence the classification of Zn
2+ as an essential micronutrient. Unlike elsewhere in the body, a healthy pancreas contains relatively high levels of the mineral
[3][9]. The predominant source of pancreatic Zn
2+ is found within the β-cells; specifically, they are contained in the dense cores of insulin secretory granules (ISGs). This presence highlights the critical nature of Zn
2+ for insulin processing and storage
[3][6][9,14].
As shown in
Figure 12, ZIP6 transports Zn
2+ from the extracellular area into the β-cell, while ZnT1 moves Zn
2+ from the cytosol towards the extracellular space. ZnT5 and ZnT6 move Zn
2+ from the cytosol into the endoplasmic reticulum (ER), while ZIP6, ZIP7, and ZIP9 perform the reverse. ZnT5 and ZnT7 transport Zn
2+ from cytosol to the Golgi apparatus and ZIP7, ZIP9, ZIP11, and ZIP13 work moving Zn
2+ out of the Golgi into the cytosol (not pictured)
[7][11].
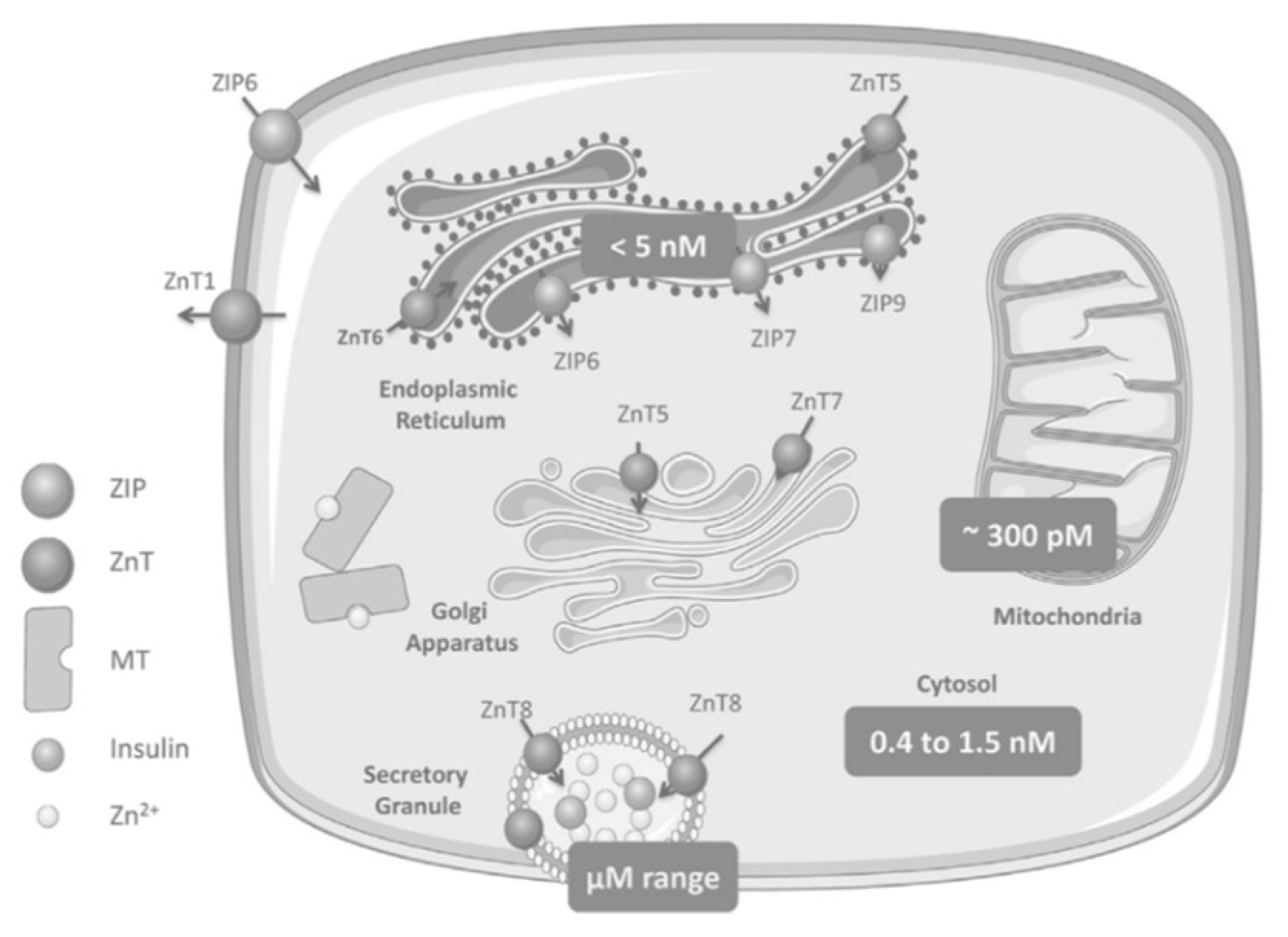
Figure 12. Zinc homeostasis in pancreatic beta cells includes multiple ZnT and ZIP transporters with ZnT8 transporters specific to the ISGs of pancreatic cells
[3][9].
ZnT8 is expressed with high specificity to pancreatic cells; it is a transmembrane protein of the ISG in islet beta and alpha cells and moves Zn
2+ from the cytosol into these cells
[14][27]. The selective site and function of ZnT8 establishes its immediate role in glucose homeostasis and insulin biology.
Within the β-cell, an inactive, single-chain preproinsulin molecule is produced in the rough endoplasmic reticulum (ER) and is subsequently cleaved to form proinsulin
[15][28]. Transporter ZnT8 imports this into the ISG where maturation occurs followed by a second cleavage generating a c-peptide along with a native insulin molecule
[3][9]. With adequate levels of Zn
2+ and insulin, a hexamer is formed, and to obtain maximum storage capacity within the secretory vesicle, the hexamerization process decreases insulin solubility and produces crystallized proinsulin
[3][15][9,28]. When blood glucose levels are high, the hexamers are converted into active monomers and expelled into the extracellular medium while concurrently freeing up a considerable concentration of Zn
2+. Implications of whether these ions act downstream to further manage the actions of insulin or proceed to other tasks independent of insulin are unclear
[3][9].
2. Zinc Transporters: Glucose Homeostasis, Insulin Resistance and Immunity
2.1. ZnT8
Due to the specificity and purpose of ZnT8 within the pancreatic β-cells, the recent literature has examined the potential therapeutic and diagnostic roles of this transporter. Huang et al. (2019) reviewed the functions of ZnT8 in diabetes, highlighting common gene polymorphisms and mutations that may increase the risk of Type 2 diabetes mellitus (T2DM) or have protective effects, respectively. Carriers of the SLC30A8 risk allele have lower insulin secretion, less conversion of proinsulin to insulin, decreased insulin sensitivity, and attenuated β-cell function. Meanwhile, other SLC30A8 mutations were linked with the therapeutic efficacy of antidiabetic drugs. The review by Nourazi et al. (2017) shared similar evidence where gene polymorphisms of ZnT8 and carriers of certain risk alleles played a part in the pathogenesis and likelihood of developing T2DM. Fukunaka and Fujitani (2018) suggested that ZnT8 levels determine the risk of T2DM development in mouse models. Electron microscopy analyzed the presence of dense ISGs in β-cells and found that ZnT8-KO mice presented irregular granules with distorted or empty cores. These mice also displayed mild glucose intolerance compared with the control group. The authors performed a pancreas perfusion experiment which indicated that insulin secretion was enhanced in ZnT8-KO mice, but the majority of the insulin produced was degraded in the liver. This indicates that the ZnT8 transporter may play a role regulating insulin clearance in the liver. The authors concluded that ZnT8 is a critical actor in insulin delivery to peripheral organs and subsequently on overall glucose metabolism.
2.2. ZIP7
While T2DM indicates major metabolic dysfunction and aberrant blood glucose levels, IR is the precursor to this disease and is marked by the body’s inability to respond properly or in a timely fashion
[5][10]. IR is induced by the distortion of accurate cell signaling and effective glucose uptake into peripheral tissues
[7][11]. ZIP7 is responsible for transporting Zn
2+ out of the ER and Golgi into the cytosol; it also controls cell signaling pathways (of note, the insulin receptor substrate-phosphoinositide-3-kinase-protein kinase B (IRS-P13K-AKT) pathway) akin to insulin, which initiates glucose uptake in skeletal muscle
[5][7][10,11]. Literature suggests that ZIP7, as linked with ER stress and cell-signaling pathways, is at the root of IR-associated distortions.
As seen in
Figure 23, ZIP7 action increases the cytosolic Zn
2+ concentration, which participates in a signaling pathway that elicits glucose mobilization and metabolism
[5][16][10,29]. In this regard, Zn
2+ acts as an insulin mimetic through the phosphorylation of AKT and the resultant mobilization of Glut4 transporters to facilitate the influx of glucose in skeletal muscle
[7][11]. In a Zn
2+-depleted cytosolic environment, ER protein folding becomes compromised, thereby activating the unfolded protein response (UPR); the rate of folding is thus reduced, but if ER stress does not resolve, apoptosis is triggered
[5][10].
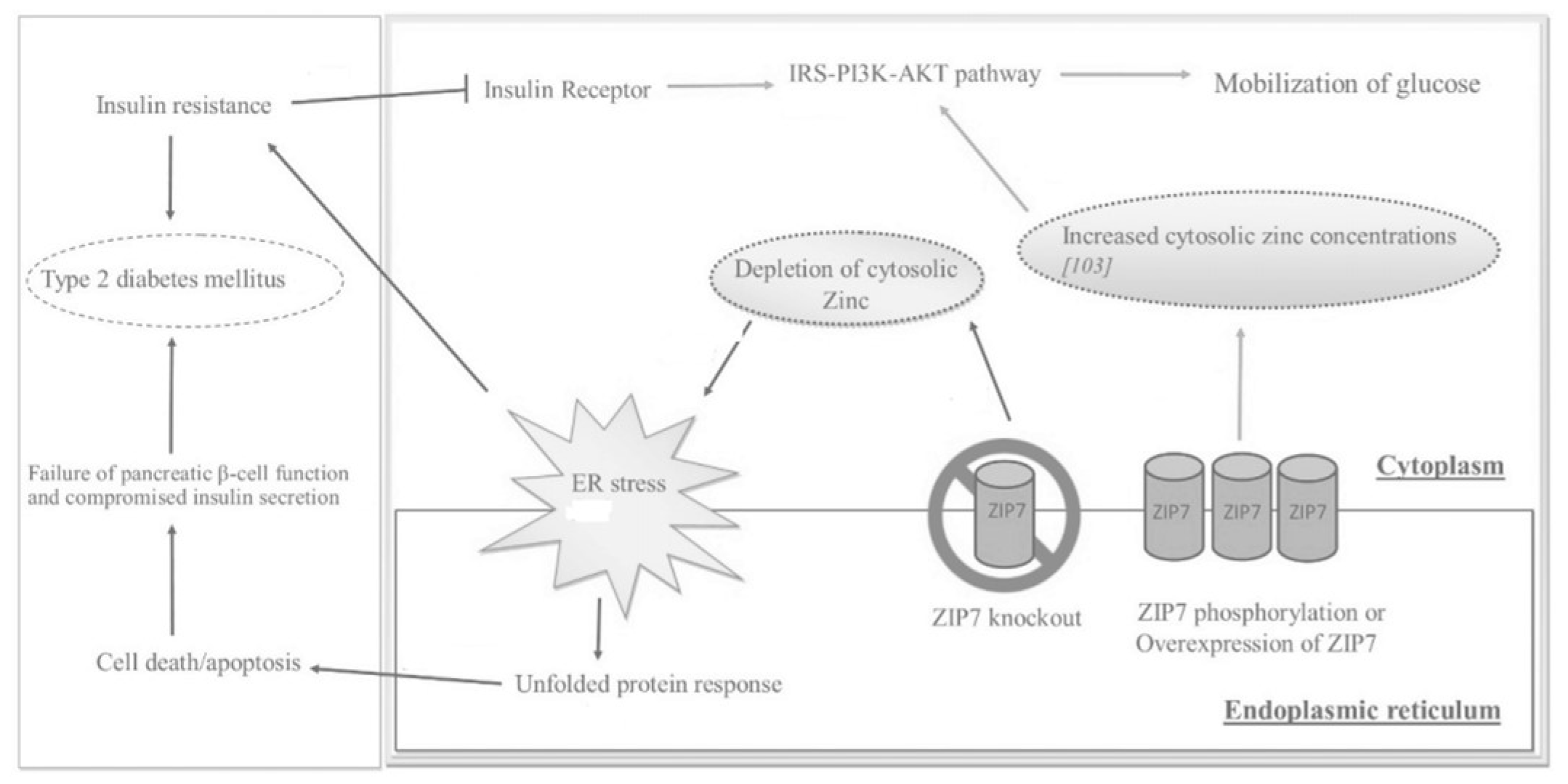 Figure 23.
Figure 23. The effects of ZIP7 on glucose mobilization, ER stress,
insulin resistance (IR
), and T2DM as depicted through various regulatory pathways. Adapted from
[5][10].
2.3. Metallotionein
MT is a potent ROS scavenger that offers significant protection against DM and DM-induced cardiovascular injury
[17][33]. Studies of Zn
2+ supplementation in diabetic mice have shown that the expression of MT is significantly induced by cellular Zn
2+ levels
[18][17][17,33]. This mechanism has been further confirmed as Zn
2+ supplementation up-regulates the expression of MT and consequently decreases diabetes-induced vascular complications
[18][17]. Research has shown that MT single nucleotide polymorphisms are related to various pathological processes, three of which are linked with a significant increase in T2DM prevalence due to reduced MT antioxidant capabilities
[11][8]. Zn
2+ effectively determines MT levels through the stimulation of responsive metal transcription factor 1 (MTF-1); this transcription factor directly regulates the expression of MT
[19][2].
2.4. Other ZnT/SLC30A Transporters
The efflux of Zn
2+ in smooth muscle cells is regulated by the ZnT1, ZnT5, and ZnT9 transporters
[12][25]. Studies have shown that levels of ZnT1 and ZnT2 are impacted by dietary intake of Zn
2+ while ZnT4 expression is not diet-dependent
[1][15]. ZnT3 transporters are predominantly located within neurons, transporting Zn
2+ to the synaptic vesicles of glutaminergic hippocampal neurons
[11][6][8,14]. ZnT3-null mice have been shown to demonstrate reduced insulin gene expression and secretion
[1][15]. The overexpression of ZnT5 and ZnT7 can prevent cellular death in hyperglycemic conditions, whereas the inhibition of these transporters is linked to an increase in apoptosis
[11][8]. ZnT5 was also found to be abundantly expressed in human endothelial cells while ZnT9 was highly expressed in human and rat hearts
[12][25]. Some studies have suggested that ZnT7 plays a redundant role of ZnT8 in the pancreas but this remains unclear as ZnT7-KO and ZnT8-KO studies have illustrated inconclusive results
[11][6][8,14].
2.5. Other ZIP/SLC39A Transporters
The ZIP1 transporter, regulated by testosterone and prolactin, is linked with rapid cellular accumulation and uptake of Zn
2+ in cells
[1][15]. ZIP1 and ZIP13 are also the dominant SLC39A transporters expressed in human endothelial tissue
[12][25]. ZIP6 impacts insulin secretion in pancreatic β-cells, whereby the down-regulation of the transporter results in dysfunctional insulin secretion in response to glucose
[20][16]. ZIP8 action increases intracellular Zn
2+ levels and may also play a role in lung epithelial cells
[1][15]. Zn
2+ that is passed to macrophages and monocytes by ZIP8 under inflammatory conditions highlights its key role in protecting against inflammation
[9][12]. ZIP13 is associated with beige adipocyte synthesis and energy metabolism through the use of Zn
2+ to inhibit adipocyte browning
[21][37]. It was found that ZIP13-KO mice produced higher levels of beige adipocytes and consequently had an improved glucose metabolism and insulin tolerance
[6][14]. ZIP14 can transport Zn
2+, iron, and manganese, with up-regulation occurring under pro-inflammatory conditions (stress, acute infection, inflammation) when there are elevated concentrations of interleukin-6 (IL-6) and NO
[1][22][15,38]. The acute phase response during inflammation will up-regulate ZIP14 for the rapid intake of plasma Zn
2+ into the organs, primarily the liver, to limit Zn
2+ availability for invading pathogens
[23][39]. Additionally, it has been noted that ZIP1, ZIP7, ZIP13, and ZIP14 are highly expressed in human heart tissue
[12][25]. A summary of zinc transporters, regulators, and effects can be found in
Table 1.
Table 1.
Zinc transporters, regulators, and effect his is a table.
| Zinc Transporter |
Regulators |
Effect |
| ZnT1 |
Metal-responsive mode of regulation; dietary intake of zinc |
Efflux of zinc in smooth muscle cells |
| ZnT2 |
Metal-responsive mode of regulation; dietary intake of zinc |
Zinc transport in vesicles and lysosomes of pancreas, kidney, testis, epithelial cells, small intestine |
| ZnT3 |
Glucose status |
Transport of zinc to synaptic vesicles |
| ZnT4 |
Unaffected by changes in dietary zinc uptake; regulated by extracellular zinc concentrations |
Transport of zinc in the trans-Golgi network and in the cytoplasmic vesicular compartment |
| ZnT5 |
Glucose status; zinc-responsive elements |
Transport of zinc into Golgi lumen for storage |
| ZnT7 |
Glucose status |
Transport of zinc to Golgi apparatus in retina, liver, epithelial cells, small intestine; may play a redundant role of ZnT8 |
| ZnT8 |
Glucose status |
Regulation of zinc in the secretory vesicles of pancreatic β-cells |
| ZnT9 |
Expressed in low levels in response to dietary intake of zinc |
Export of zinc out of myocytes; efflux of zinc in smooth muscle cells |
| ZIP1 |
Testosterone and prolactin |
Uptake of zinc into cells |
| ZIP6 |
Estrogen stimulation, glucose status |
Down-regulation leads to poor insulin secretion |
| ZIP7 |
Glucose status |
Increases cytosolic zinc concentrations that participate in glucose mobilization and metabolism |
| ZIP8 |
Glucose status, TNF-∝ in lung epithelial cells |
Increases intracellular zinc levels |
| ZIP13 |
Gene mutation leads to loss of function |
Inhibition of adipocyte browning |
| ZIP14 |
Acute phase response during inflammation; IL-6 |
Rapid intake of plasma zinc into the organs |
3. Conclusions
The full extent of zinc’s role in the molecular mechanisms involved in the pathogenesis and pathophysiology of many chronic diseases, including diabetes, has not yet been fully uncovered
[8][4]. Yet, the current literature acknowledges the deleterious effects of dysregulated Zn
2+ homeostasis in diabetes and its role in the development of both micro-vascular and macro-vascular complications
[19][8][12][23][2,4,25,39]. Inadequate levels of Zn
2+ result in impaired antioxidant functioning, cytokine over-expression, chronic inflammation, ROS accumulation and oxidative damage, distorted lipid and glucose metabolism, ER stress, β-cell defects and apoptosis, IR, endothelial dysfunction, and the evolution of cardiovascular complications
[9][12]. Zn
2+ depletion is now a hallmark characteristic of DM and is at the core of many pathogenic changes driven by hyperglycemia-induced oxidative stress and DNA damage. As such, the clinical management of Zn
2+ nutrition over the course of the condition is paramount in maintaining Zn
2+ homeostasis and the subsequent regulation of MT and Zn
2+-transporter expression
[24][48].
Future research is required for the expression of Zn
2+ transporters and MT as biomarkers for the Zn
2+ status, along with additional analyses of EVs from liquid biopsies
[12][25]. More prospective cohort studies are needed in order to ascertain whether Zn
2+ supplementation is indeed an effective agent in the prevention of diabetes onset
[6][14]. And further investigation of Zn
2+ supplementation in humans is necessary such that a clinical standard of care, relative to Zn
2+ therapy, may be implemented jointly with other treatments.
Although the complete mechanistic relationship between the Zn
2+ status and the occurrence of chronic disease remains obscured, studies have established that Zn
2+ deficiency, by the induction of inflammation and oxidative stress, promotes the onset/progression of many conditions (DM, metabolic syndrome, obesity, cancer, kidney disease, neurodegeneration, atherosclerosis, CVDs)
[8][6][23][4,14,39]. The comprehensive effect of Zn
2+ homeostasis on the immune system is undeniable; this suggests that restoring normal levels of the metal through supplementation may prove to be an effective therapeutic measure, not only for diabetes, but for overall human health.

 Figure 23. The effects of ZIP7 on glucose mobilization, ER stress, insulin resistance (IR), and T2DM as depicted through various regulatory pathways. Adapted from [5][10].
Figure 23. The effects of ZIP7 on glucose mobilization, ER stress, insulin resistance (IR), and T2DM as depicted through various regulatory pathways. Adapted from [5][10].
