Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Lida Fuentes-Viveros.
Fleshy fruits are characterized by having a developmentally and genetically controlled, highly intricate ripening process, leading to dramatic modifications in fruit size, texture, color, flavor, and aroma. Climacteric fruits such as tomato, pear, banana, and melon show a ripening-associated increase in respiration and ethylene production and these processes are well-documented. Recent studies have shown that non-climacteric fruit development and ripening, involves the coordinated action of different hormones, such as abscisic acid (ABA), auxin, gibberellins, ethylene, and others.
- fruit quality parameters
- phytohormones
- ethylene
- auxin
- abscisic acid (ABA)
- brassinosteroids
- jasmonic acid
- grape
- strawberry
- raspberry
1. Introduction
Angiosperms produce different fruit categories that evolved to best suit seed protection and aid in seed dispersal towards the final stages of fruit ripening. Botanically, fleshy fruits are very varied in the way they develop. For example, in grape (Vitis spp.) and tomato (Solanum lycopersicum L.), fruit is from the result of a developed ovary, whereas, in strawberry (Fragaria spp.), apple (Malus × domestica Borkh.), and pineapple [Ananas comosus (L.) Merr.], it results from the accessory tissue external to the carpels, the receptacle [1[1][2],2], or formed from a series of ovaries (drupelets) attached to a receptacle as is the case of fruits belonging to the genus Rubus [3,4,5,6,7,8,9,10][3][4][5][6][7][8][9][10]. Evolutionary studies have revealed that both dry and fleshy fruit share some common developmental mechanisms, and that fleshy fruit has evolved from ancestral dry fruit-producing species [11]. Whether fleshy or dry fruit, the final fruit involves a progression of specific steps, namely, fruit set, fruit development, and fruit ripening and senescence [2,12][2][12]. In general, fruit ripening is marked by very important phase changes that result in the conversion of less appetizing green fruit into a highly palatable, aromatic, colored, and nutritionally rich fruit.
Fruits are characterized by having a developmentally and genetically controlled development and ripening process, leading to both physiological and biochemical changes [1,13,14,15,16,17][1][13][14][15][16][17]. These changes generally occur through tightly regulated events such as, (i) colour change, by the decrease of chlorophyll content, with anthocyanin and/or carotenoid accumulation, (ii) changes in modification of cell wall structure and cell turgor that result in the change of fruit texture, (iii) increases of different metabolites (acids, sugars, volatiles, among others) that impact flavor, aroma, and nutritional quality, and (iv) increased susceptibility to pathogen attack at the later stages of ripening that result in fruit spoilage [1,2,14,18][1][2][14][18].
Based on their ethylene evolution and respiration pattern, fleshy fruits are categorized as climacteric and non-climacteric, according to the regulatory mechanisms underlying the ripening process [19,20][19][20]. Climacteric fruits or ethylene-dependent fruits have the capability to ripen after harvest with the help of ethylene production [21]. Climacteric fruits such as banana (Musa spp.), tomato, avocado (Persea americana Mill.), and apple are characterized by a dramatic increase in respiration and ethylene evolution during the onset of ripening [1,2,13][1][2][13]. Non-climacteric fruits are not capable to ripen after removal from the parent plant, whereas climacteric fruits are [21]. Those fruits like strawberry, grape, raspberry (Rubus idaeus L.), and citrus (Citrus spp.) are defined by the absence of an ethylene-related respiratory peak and do not show a climacteric rise in ethylene evolution [1,2,13,22][1][2][13][22]. Despite the above, the classification of fruits as either climacteric or non-climacteric is not obvious, because some fruits, like melons (Cucumis spp.), can display both climacteric and non-climacteric behaviors [23]. Furthermore, there are climacteric and suppressed-climacteric plum varieties, whose ability to respond to ethylene could be affected [24]. More complicated regulation has been observed in kiwifruit, where the first stage of ripening is not dependent on ethylene whereas the second stage is [25]. For other fruit such as strawberry and raspberry, typically classified as non-climacteric, current molecular studies suggest a certain role of ethylene in ripening. It was reported that transcript levels of ethylene receptors increase at the onset of ripening in strawberry [26], and transcript levels of ethylene biosynthesis increase with ripening progression in raspberry [10].
Independent of classification as climacteric or not, color and textural changes are the main modifications observed during fruit ripening together with changes of organic acids, sugars, and volatile compounds [12,14][12][14] that contribute to fruit flavor, particularly by adjusting the equilibrium between organic acids and sugar [27,28][27][28]. Modifications in fruit size and color are considered important parameters for the ripening-stage differentiation of many fruits, including the non-climacteric fruits such as grape, strawberry, and raspberry [10,29,30,31][10][29][30][31]. The red or purple color seen in these fruits are mainly due to the accumulation of anthocyanins. These compounds are water-soluble pigments, synthesized as products of the phenylpropanoid pathway in the cytosol and localized in vacuoles during ripening and stress responses, and have been widely studied in different fruit species including raspberry and blackberry, due to their health benefits [32,33,34,35,36,37,38,39][32][33][34][35][36][37][38][39].
Postharvest fruit softening contributes to the deterioration of fruit quality and makes postharvest management difficult with fleshy fruit. The fungus Botrytis cinerea Pers.:Fr. has often been reported as the main pathogen causing rapid fruit decay, especially in grape, strawberry, and raspberry [38,39,40][38][39][40]. Cell wall changes accompanied by an intense decrease in the content and the degree of methyl-esterification of pectin during ripening have been reported in different raspberry cultivars [41], Chilean strawberry [Fragaria chiloensis (L.) Mill.] and cultivated strawberry (Fragaria × ananassa Duch.) [30,42][30][42].
Changes in flavor and taste are directly reliant on the sugar–acid balance and contents of the fruit, which is significantly important for consumers [27,28][27][28]. For instance, too much acid results in a tart and unpalatable fruit; conversely, too little results in bland and insipid fruit. Acid levels, expressed as titratable acidity (TA), changes in the starch breakdown, and soluble sugar increase have been used as indicators of taste [43] and a critical index of fruit ripening [28]. Also, the flavor can be determined by the presence of molecules such as anthocyanins and tannins, that grant slight and pleasing astringency [28]. Aroma, one of the most valued attributes of raspberry [44] and strawberry [45,46][45][46] fruits, is a parameter that depends on a number of factors such as concentration, combination, and the perception threshold of different volatile compounds [45[45][46][47],46,47], and the main aroma components include lipid-derived compounds, phenolic derivatives, amino acid-derived compounds, and mono- and sesquiterpenes [47].
2. Hormonal Regulation of Ripening in Non-Climacteric Fruit
Conventionally, non-climacteric fruits have been classified as a separate group that did not show the typical climacteric ripening pattern. However, studies including comparative genomic data analysis carried out in tomato and hot pepper (Capsicum spp.) as climacteric and non-climacteric fruit models, respectively, show that the expression of genes encoding for transcription factors such as non-ripening (NOR), tomato AGAMOUS-like 1 (TAGLI) and ripening inhibitor (RIN), and for ethylene signaling pathway-related components are common steps in both fruit categories [54][48]. Also, the identification of MADS-box genes in these two categories of fruit suggests that at least some molecular regulatory processes of fruit ripening are common between climacteric and non-climacteric fruits [55][49]. Plant hormones are widely known to be regulators of fruit development and ripening [1,2,3,56,57,58][1][2][3][50][51][52]. Recent evidence has indicated that the shared action of three hormones, namely, auxin, cytokinin, and gibberellins, contributes to normal fruit growth even in the absence of fertilization, a process known as parthenocarpy; application of these hormones alone starts fruit development in many species [2,59,60,61[2][53][54][55][56][57],62,63], suggesting that communication between these hormones is necessary for fruit set and fruit growth. An overview of phytohormones involved in non-climacteric fruit development and ripening (especially of grape, strawberry, and raspberry) and their possible crosstalk is described below (Figure 1).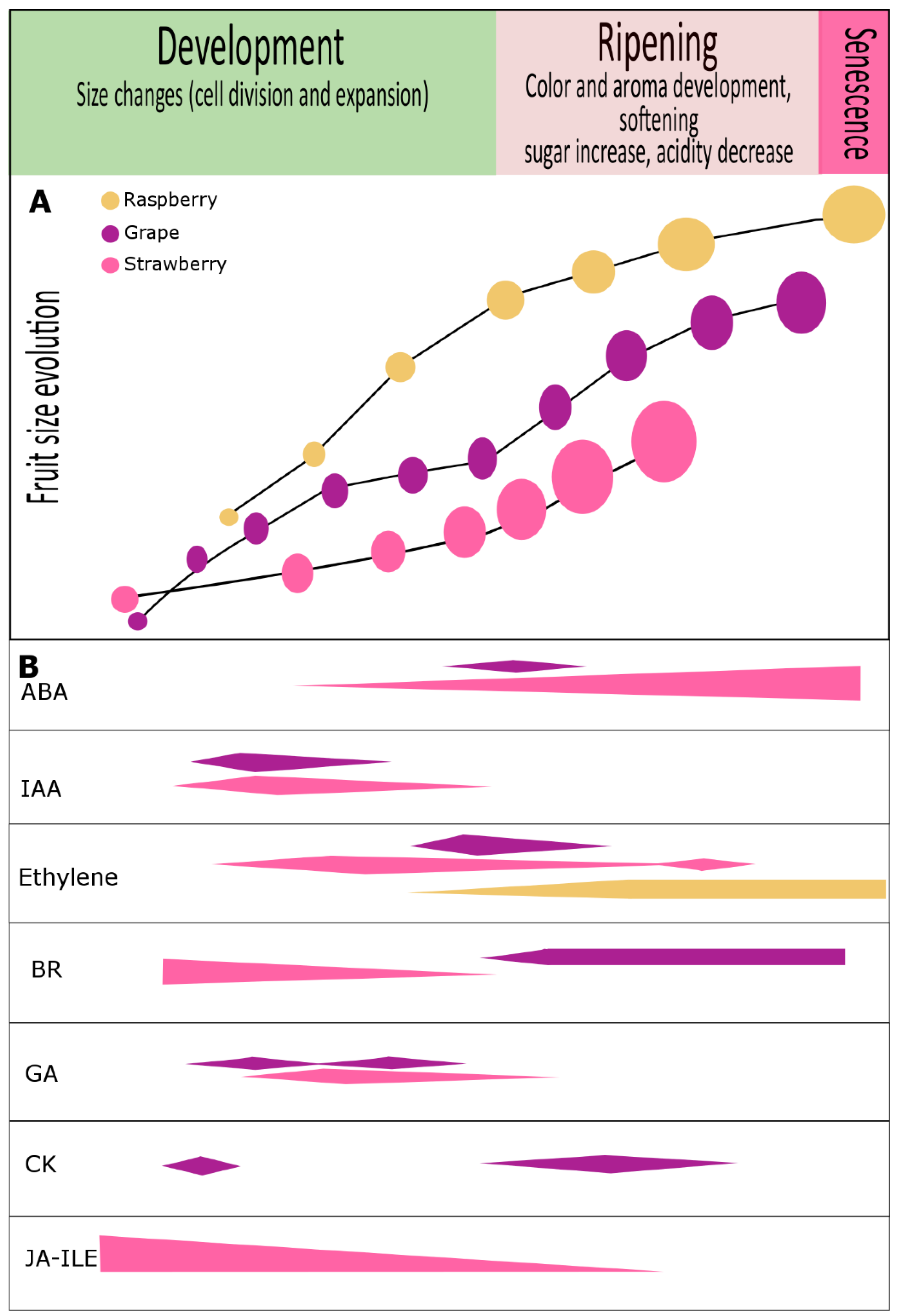
Figure 1. Phytohormone content evolution during development and ripening of grape, strawberry and raspberry fruits. (A) Developmental trends according to fruit size in grape (purple) [203][58], strawberry (pink) [18,51][18][59] and raspberry (yellow ochre) [53][60]. (B) Variations in hormone levels are shown for abscisic acid (ABA), auxin (IAA), ethylene, brassinosteroids (BR), gibberellins (GA), cytokinins (CK), and jasmonic acid isoleucine conjugate (JA-Ile). In grape, numerous reports have revealed an important role for auxins, cytokinins, and gibberellins (GA3) associated with cell division and fruit set. At véraison, a noticeable increase in abscisic acid levels was reported to be crucial for ripening progression, but during ripening a single and constant hormonal pattern is not the rule [203][58]. In strawberry, abscisic acid is thought to be important, but the roles of other hormones including IAA, BR, GA1, GA3, GA4, JA-Ile, and ethylene, were also suggested to be involved in fruit development. Although raspberry fruit has been described as non-climacteric fruit, ethylene can be detected at the onset of ripening and continues to increase until full ripening [3,10,53][3][10][60]. There are no reports regarding other hormones in raspberry, although we could detect the presence of auxin in green stages and our transcriptome analysis has shown differential expression of phytohormone-related genes during fruit development and ripening [204][61].
3. Hormone Crosstalk
A single hormone can regulate various processes, and at the same time, multiple hormones could impact a single process as well [205,206][62][63]. Some pieces of evidence for auxin–ethylene crosstalk was reported in Capsicum, and the expression of the CsGH3 gene was upregulated by ethylene application [207][64]. Similarly, the upregulation of the expression of GH3 family-related genes by ABA and ethylene application during fruit ripening in grape and by other phytohormones in tomato suggests that auxin can crosstalk with ethylene, ABA, and other phytohormones [2,57,208,209][2][51][65][66]. ABA promoted fruit ripening by affecting ethylene biosynthesis in fleshy fruits [210,211][67][68]. For example, the exogenous application of ABA increased the expression of ethylene biosynthesis-related genes, such as ACS2, ACS4, and ACO1, in tomato. Conversely, exogenous treatment with the ABA inhibitor fluridone showed downregulation of these genes, suggesting that ABA could regulate the ethylene biosynthetic pathway and vice versa in climacteric fruit such as tomato [2,212][2][69]. Contrary to that of climacteric fruit such as tomatoes, where maximum ABA levels were observed preceding ethylene production [213[70][71],214], ethylene levels in grape remained low at the onset of ripening [56][50].
Data from several transcriptomic analyses, including our data on raspberry [204][61], suggest a coordinated role of different phytohormones during fruit development and ripening in non-climacteric fruit such as grape [104,215][72][73] and strawberry [31,216][31][74]. However, an in-depth analysis is still required to fully elucidate the role of phytohormone crosstalk in non-climacteric fruit ripening. When compared with climacteric fruit, much less information is available on phytohormone crosstalk in non-climacteric fruit. In the following sections, we briefly summarize the available literature on plant hormone crosstalk during fruit development of grape, strawberry and raspberry fruit (Figure 2).
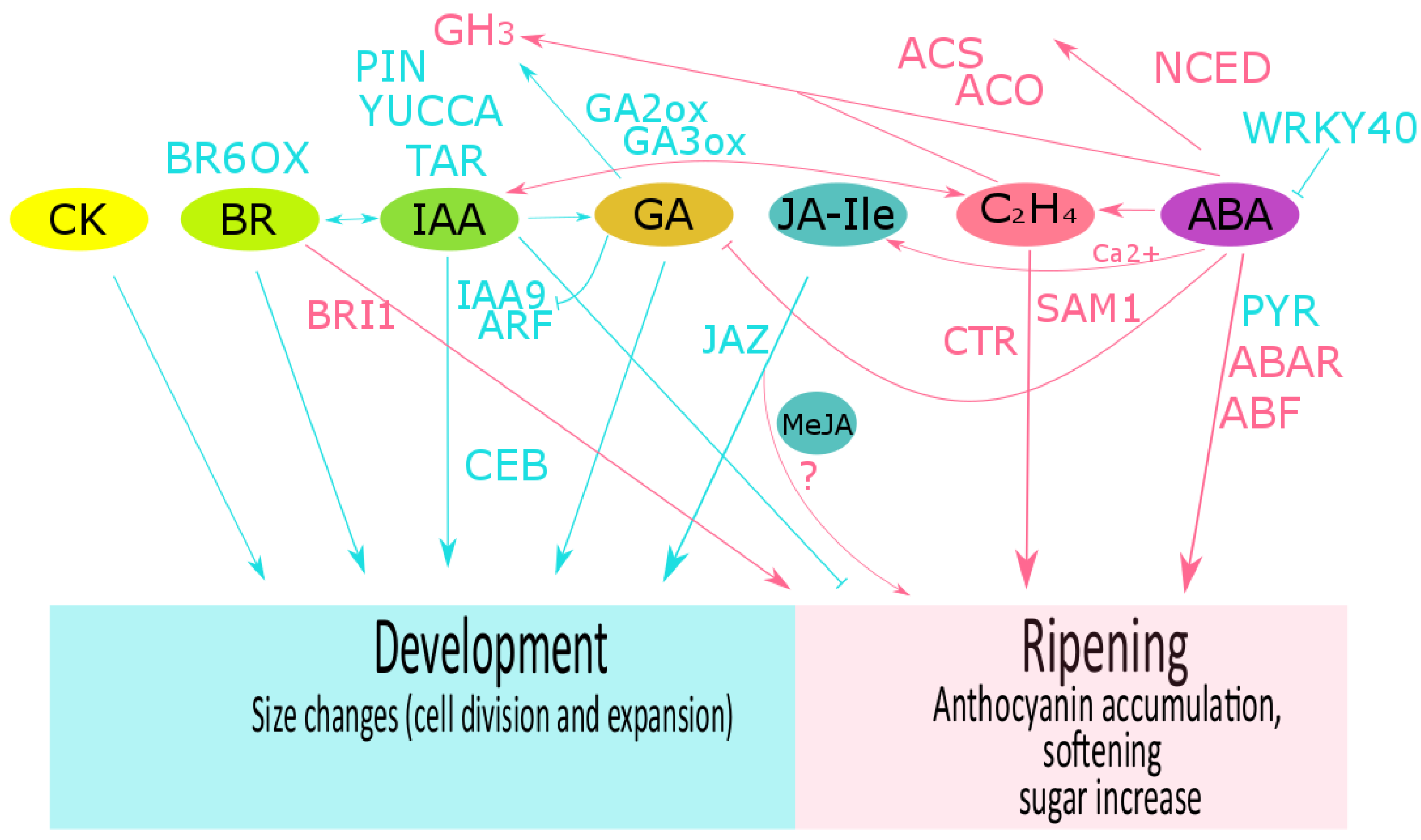

Figure 2. Phytohormone crosstalk described in grape, strawberry and raspberry fruits. The role of hormones and their related components during fruit development (cyan color) and during ripening (pink color) are shown for abscisic acid (ABA), auxin (IAA), ethylene (C2H4), brassinosteroids (BR), gibberellins (GA), cytokinins (CK), and jasmonic acid isoleucine conjugate (JA-Ile). In many fruits, development is controlled by a balance between GA and auxin. In grape, GA downregulates VvIAA9 and VvARF7 expression and then auxin transport, and upregulates VvGH3 expression. In turn, auxins could promote fruit set and biosynthesis of gibberellins. IAA and BRs crosstalk has been suggested as an important mechanism to control fruit growth. During grape ripening, an interaction between ethylene and auxin has been described, and calcium-ABA-JA interplay could regulate the expression of flavonoid biosynthesis. In strawberry, ABA, auxin and GA crosstalk regulates the transition of development to ripening. Methyl jasmonate (MeJA) application at the onset of ripening stimulates ethylene and anthocyanin biosynthesis. However, the JA-Ile content has been suggested as important for fruit development. FaNCED1 downregulation by MeJA application suggests an antagonistic role of JA on ABA biosynthesis. In raspberry, exogenous IAA and MeJA regulate the anthocyanin content. TAR: tryptophan aminotransferase; YUCCA: flavin-containing monooxygenases; PIN: auxin transporter (PIN-FORMED protein); GH3: IAA-amido synthetase; ARF: auxin response factor; CEB1: transcriptional factor cell elongation bHLH protein; NCED: 9-cis-epoxycarotenoid dioxygenase; PYR1: ABA receptor (pyrabactin resistant); FaABAR: ABA receptor; ABF2: ABA transcriptional factor; GA-n-ox: gibberellin n-oxidase; ACS: 1-aminocyclopropane-1-carboxylic acid synthase; ACO: 1-aminocyclopropane-1-carboxylic acid oxidase; SAMS1: S′adenosyl-l-methionine synthase; ETR: ethylene receptor; CTR1: constitutive triple response gene; ERS1: ethylene response sensor; EIN: ethylene insensitive; JAZ: jasmonate zim-domain protein; BR6OX: BR 6-oxidase; BRI1:BR receptor. More details in the text.
3.1. Grape
Transcriptional analysis of grape berries after application of naphthalene acetic acid (NAA) one week before véraison showed that ABA biosynthesis- and perception-associated genes were downregulated, while ethylene biosynthesis-associated ones were induced [104][72]. In addition, the interaction between ethylene and auxin has been suggested as a mechanism of grape ripening control [118][75]. The expression of several TAR genes that encodes for the enzyme that converts tryptophan to indole-3-pyruvate, the first step of IAA biosynthesis, was induced by the application of ethephon [118][75]. The induction of TAR genes was complemented by increased IAA and IAA-Asp concentrations, suggesting that elevated concentrations of ethylene at the onset of ripening might lead to increased production of IAA in grape berry ripening [118][75].
The developmental phase in many fruits was reported to be controlled by a hormonal balance between GA and auxin [203][58]. Auxin induces the generation of parthenocarpy. Treatment with GA at the pre-bloom stage of berries showed an upregulation of the GA signaling gene VvDELLA, together with a decrease of the expression of the genes encoding for negative regulators of fruit set initiation, i.e., AUX-IAA protein, VvIAA9 and auxin response factor (ARF) VvARF7. Also, the upregulation of VvGH3.2 and VvGH3.3 expression, without significant effects on VvYUC2 and VvYUC6 expression, was reported. This suggests that GA signaling is associated with IAA signaling via VvDELLA during parthenocarpy in grape [160][76]. Exogenous application of the auxin analogue 4-chlorophenoxyacetic acid (4-CPA) to ovaries of ‘Fenghou’ grape, was reported to promote fruit set, depending on subsequent biosynthesis of gibberellin GA3 [217][77].
Calcium (Ca2+) has many roles in plants serving as a second messenger, and in cell wall polysaccharide interactions, crucial in stress responses, cell wall growth, and remodeling, and plant tissue development [218,219,220][78][79][80]. However, its molecular implication on phytohormone crosstalk regulation is still not very clear. Studies of ABA and MeJA applied alone or in combination with calcium to grape suggested a calcium-hormone interplay that regulated the expression and activity of flavonoid biosynthetic enzymes [221][81].
IAA and BRs have been described as key regulators that determine the growth of several plants, influencing cell division and elongation in various developmental contexts [222][82]. Despite the reports available about the role of BRs in fruit quality, their crosstalk with other phytohormones can only be suggested based on transcriptomic analyses. Comparison of transcriptomic analyses between varieties of small and large grape berries have shown that differentially expressed transcripts were mainly related to auxin, ABA, ethylene, but also to BRs and gibberellins [215][73]. The comparison between small and large grape berries during fruit development revealed significant differences in softening rate, firmness, and sugar accumulation. The transcriptional dataset showed DEGs related to auxin, ABA and the ethylene hormone pathway between varieties of small and large grape berries [215][73]. The same study showed the specific regulatory motifs related to bZIP, bHLH, AP2/ERF, NAC, MYB, and MADS-box transcription factors in the cis-regulatory element of the promoter regions of DEGs related to fruit texture, aroma, and flavor, and hormones, observing differences in the promoter regions between small and large grape berries [215][73].
Exogenous application of GA3 and IAA to grape reduced polar auxin transport, but only GA3 treatment decreased VvPIN transcript abundance. The GA biosynthesis blocking allowed increased IAA polar auxin transport, suggesting that polar auxin transport depends on GA content [215][73]. In tomato, molecular studies revealed that an auxin response factor (SlARF7) was involved in the regulation of the crosstalk between auxin and GA, and silencing of this gene caused parthenocarpic fruit formation due to augmented auxin and GA responses, and further suggest that ARF7 acts as a changer of the GA response in early fruit development as has been reported in Arabidopsis and tomato [61,223][55][83]. However, the potential role of ARF as a GA-response regulator during grape development still needs to be investigated.
3.2. Strawberry
In strawberry fruit, expression analysis showed that auxin and ABA are the main hormones responsible for the transition from development to ripening stages [224][84]. Auxins are responsible for the development of the receptacle and, at the same time, prevent ripening by repressing key ripening-related genes. ABA regulates the expression of the majority of genes involved in ripening, while ethylene and gibberellins do not seem to play a noticeable role during maturation [224][84]. After harvest of strawberry, exogenous IAA delayed strawberry ripening, while exogenous ABA treatment had the contrary effect. However, the combined treatment did not show any of these effects on the postharvest ripening of the strawberry fruits [225][85]. A comparison of transcriptomes of fruit under the individual or combined treatments revealed that there were differentially-expressed unigenes in response to the ABA and IAA combination treatment [225][85]. Exogenous IAA application upregulated IAA signaling-related genes such as AUX/IAAs and ARFs, and downregulated cell wall degradation-, sucrose and anthocyanin biosynthesis-related genes. Conversely, ABA-induced the expression of genes related to fruit softening and signaling pathways as that encoding for the S-phase kinase-associated protein (SKP1) component of the Skp1-Cullin1-F-box (SCF) complex that facilitates ubiquitin-mediated protein degradation [224][84]. The expression of a C-type MADS-box gene in strawberry [SHATTERPROOF-like (FaSHP)] was downregulated by auxin and upregulated by ABA [55][49]. In addition, its promoter presented responsive elements to auxin and ABA, explaining how FaSHP could be controlled by these two hormones [55][49].
Regarding the possible role of calcium in hormone crosstalk in strawberry fruit, the application of calcium in combination with auxin (as NAA) reduced the transcript level of the cell wall modifying-related genes FcPG1, FcPL and FcEG1 (encoding for polygalacturonase, pectate lyase and endoglucanase, respectively) [226][86]. These results suggest that the auxin repressor role in strawberry ripening could be reinforced by calcium, although more research is needed to clarify this interaction.
Recently, interactions and regulation between auxin, GA and ABA during fruit development and ripening of F. vesca fruit have been reported [227][87]. This study reported that the increase in the GA content at the early stages could antagonize the inhibitory effect of ABA, whereas the quick drop of ABA during the early stages of fruit development guarantees fruit growth induction by GA [227][87]. The ABA catabolism gene FveCYP707A4a was reported as an important crosstalk point for auxin, GA and ABA, regulating the transition from the early growth phase to the ripening phase [227][87].
MeJA application at the onset of ripening, especially at the white stage, stimulated ethylene biosynthesis by an increase in ACO activity [228][88]. In F. chiloensis fruit, the application of MeJA increased the expression of both ACO and ACS genes suggesting that several ripening effects triggered by exogenous MeJA could be mediated by ethylene [182][89]. MeJA treatment of climacteric fruit such as apple enhanced the expression of MdMYC2, a gene encoding a transcription factor involved in the JA signaling pathway. The MdMYC2 directly bound to the promoters of MdACS1 and MdACO1 genes enhanced their transcription, and then increased ethylene biosynthesis [229][90]. However, the potential role of the MYC2 transcription factor on ethylene and MeJA crosstalk must still be elucidated in strawberry. With regard to JA-ABA crosstalk in strawberry, the JA pathway could act antagonistically with ABA for anthocyanin accumulation in strawberry fruit. We reported an increase in anthocyanin content, with an associated decrease in ABA levels, after MeJA treatment, going along with FaNCED1 downregulation, suggesting an antagonistic association from the JA to the ABA pathway in strawberry [51][59].
3.3. Raspberry
With regard to hormonal crosstalk in raspberry, recent studies suggested that anthocyanin metabolism is regulated by the interplay of multiple hormonal signs, including IAA and MeJA [186][91]. This study showed that IAA downregulated and MeJA clearly upregulated the expression of the genes encoding for transcription factor MYB10 and anthocyanin synthase (ANS), two genes related to anthocyanin biosynthesis, suggesting an opposite effect of both hormones in the regulation of anthocyanin accumulation in fruits [186][91]. Although there is still a lot to study in terms of raspberry fruit ripening regulation, our de-novo assembly analysis during different developmental stages showed that the DEGs included transcripts related to synthesis and response to ABA, enzymes with GH3 activity, transmembrane transporter for influx and efflux of auxin, proteins related to response to auxin, proteins related to BR biosynthesis and response, and proteins related to ethylene biosynthesis and perception [204][61]. All this information suggests a role for different hormones during raspberry development. In our experiments we have observed an increase of ethylene during the IAA treatment of raspberry [45]; however, the possibility of a stress effect of the treatment should not be ruled out.
The loss of membrane integrity during the decline in fruit quality has been associated with 1-phospholipase D (PLD), a phospholipid-degrading enzyme involved in initiating membrane catabolic events that are highly active in berries such as strawberry and raspberry [230,231][92][93]. The pre-harvest application of aqueous spray containing hexanal (HC), an enhancer of fruit shelf-life, during fruit development showed significant downregulation of transcript levels of three PLD encoding genes and its associated enzymatic activity, as well as upregulation of the expression of the genes related to calcium-binding protein such as annexin and calmodulin-binding transcription activators [231][93]. All these changes were associated with the significant increase of the pulling force necessary to detach the berry from the receptacle and abnormal calcium crystalline depositions on the epidermal drupelet [231][93]. These antecedents suggest potential crosstalk between hexanal, phospholipase D activity and calcium in delaying fruit softening and in prolonging the storage life of raspberry.
References
- Cherian, S.; Figueroa, C.R.; Nair, H. ‘Movers and shakers’ in the regulation of fruit ripening: A cross-dissection of climacteric versus non-climacteric fruit. J. Exp. Bot. 2014, 65, 4705–4722.
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2014, 65, 4561–4575.
- Blanpied, G.D. A study of ethylene in apple, red raspberry and cherry. Plant Physiol. 1972, 29, 627–630.
- Burdon, J.N.; Sexton, R. The role of ethylene in the shedding of red raspberry fruit. Ann. Bot. 1990, 66, 111–120.
- Burdon, J.N.; Sexton, R. Fruit abscission and ethylene production of red raspberry cultivars. Sci. Hortic. 1990, 43, 95–102.
- Burdon, J.N.; Sexton, R. Practical implications of differences in the ethylene production of Rubus fruits. Acta Hortic. 1994, 368, 884–892.
- Perkins-Veazie, P.M.; Nonnecke, G. Physiological changes during ripening of raspberry fruit. J. Hortic. Sci. 1992, 27, 331–333.
- Iannetta, P.P.M.; van den Berg, J.; Wheatley, R.E.; McNicol, R.J.; Davies, H.V. The role of ethylene and cell wall modifying enzymes in raspberry (Rubus idaeus) fruit ripening. Physiol. Plant. 1999, 105, 338–347.
- Pritts, M.P. Raspberries and related fruits. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Kent, UK, 2003.
- Fuentes, L.; Monsalve, L.; Morales-Quintana, L.; Valdenegro, M.; Martínez, J.P.; Defilippi, B.G.; González-Agüero, M. Differential expression of ethylene biosynthesis genes in drupelets and receptacle of raspberry (Rubus idaeus). J. Plant Physiol. 2015, 179, 100–105.
- Knapp, S. Tobacco to tomatoes: A phylogenetic perspective on fruit diversity in the Solanaceae. J. Exp. Bot. 2002, 53, 2001–2022.
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 2013, 4, 79.
- Giovannoni, J. Molecular biology of fruit maturation and ripening. Ann. Rev. Plant Physiol. Plant. Mol. Biol. 2001, 52, 725–749.
- Giovannoni, J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16, S170–S180.
- Giovannoni, J.J. Fruit ripening mutants yield insights into ripening control. Curr. Opin. Plant Biol. 2007, 10, 283–289.
- Giovannoni, J.; Nguyen, C.; Ampofo, B.; Zhong, S.; Fei, Z. The epigenomCand transcriptional dynamics of fruit ripening. Annu. Rev. Plant Biol. 2017, 68, 61–84.
- Graham, J.; Simpson, C. Chapter 14: Developmental Transitions to Fruiting in Red Raspberry. In The Genomes of Rosaceous Berries and Their Wild Relatives; Hytönen, T., Graham, J., Harrison, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 199–212.
- Symons, G.M.; Chua, Y.J.; Ross, J.J.; Quittenden, L.J.; Davies, N.W.; Reid, J.B. Hormonal changes during non-climacteric ripening in strawberry. J. Exp. Bot. 2012, 63, 4741–4750.
- Li, C.; Jia, H.; Chai, Y.; Shen, Y. Abscisic acid perception and signaling transduction in strawberry: A model for non-climacteric fruit ripening. Plant Signal. Behav. 2011, 12, 1950–1953.
- Bouzayen, M.; Latché, A.; Nath, P.; Pech, J.C. Mechanisms of fruit ripening. In Plant Developmental Biology–Biotechnological Perspectives; Pua, E.C., Davey, M.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 1.
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit ripening phenomena-An overview. Crit. Rev. Food. Sci. Nutr. 2007, 47, 1–19.
- Bapat, V.A.; Trivedi, P.K.; Ghosh, A.; Sane, V.A.; Ganapathi, T.R.; Nath, P. Ripening of fleshy fruit: Molecular insight and the role of ethylene. Biotechnol. Adv. 2015, 28, 94–107.
- Fernández-Trujillo, J.P.; Obando-Ulloa, J.M.; Martínez, J.A.; Moreno, E.; GarcíaMas, J.; Monforte, A.J. Climacteric and non-climacteric behavior in melon fruit. 2. Linking climacteric pattern and main postharvest disorders and decay in a set of near-isogenic lines. Postharvest Biol. Technol. 2008, 50, 125–134.
- Abdi, N.; McGlasson, W.B.; Holford, P.; Williams, M.; Mizrahi, Y. Responses of climacteric and suppressed-climacteric plums to treatment with propylene and 1methylcyclopropene. Postharvest Biol. Technol. 1998, 14, 29–39.
- McAtee, P.A.; Richardson, A.C.; Nieuwenhuizen, N.J.; Gunaseelan, K.; Hoong, L.; Chen, X.; Atkinson, R.G.; Burdon, J.N.; David, K.M.; Schaffer, R.J. The hybrid non-ethylene and ethylene ripening response in kiwifruit (Actinidia chinensis) is associated with differential regulation of MADSbox transcription factors. BMC Plant Biol. 2015, 15, 304.
- Trainotti, L.; Pavanello, A.; Casadoro, G. Different ethylene receptors show an increased expression during the ripening of strawberries: Does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits? J. Exp. Bot. 2005, 56, 2037–2046.
- Chaimanee, P.; Suntornwat, O. Changes in carbohydrate content during fruit ripening-a new approach of teaching of carbohydrate chemistry in biochemistry course. Biochem. Educ. 1994, 22, 101–102.
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in Organic Acid Profiles During Fruit Development and Ripening: Correlation or Causation? Front. Plant Sci. 2018, 9, 1689.
- Vicente, A.R.; Ortugno, C.; Powell, A.L.; Greve, L.C.; Labavitch, J.M. Temporal sequence of cell wall disassembly events in developing fruits. 1. Analysis of raspberry (Rubus idaeus). J. Agric. Food Chem. 2007, 55, 4119–4124.
- Figueroa, C.R.; Pimentel, P.; Dotto, M.C.; Civello, P.M.; Martínez, G.A.; Herrera, R.; Moya-León, M.A. Expression of five expansin genes during softening of Fragaria chiloensis fruit. Effect of auxin treatment. Postharvest Biol. Technol. 2009, 53, 51–57.
- Estrada-Johnson, E.; Csukasi, F.; Pizarro, C.M.; Vallarino, J.G.; Kiryakova, Y.; Vioque, A.; Brumos, J.; Medina-Escobar, N.; Botella, M.A.; Alonso, J.M.; et al. Transcriptomic Analysis in Strawberry Fruits Reveals Active Auxin Biosynthesis and Signaling in the Ripe Receptacle. Front. Plant Sci. 2017, 8, 889.
- Mullen, W.; McGinn, J.; Lean, M.E.; MacLean, M.R.; Gardner, P.; Duthie, G.G.; Yokota, T.; Crozier, A. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J. Agric. Food Chem. 2002, 50, 5191–5196.
- Liang, Z.; Wu, B.; Fan, P.; Yang, C.; Duan, W.; Zheng, X.; Liu, C.; Li, S. Anthocyanin composition and content in grape berry skin in Vitis germplasm. Food Chem. 2008, 111, 837–844.
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779.
- Hyun, T.K.; Lee, S.; Kumar, D.; Rim, Y.; Kumar, R.; Lee, S.Y.; Lee, C.H.; Kim, J.Y. RNA-seq analysis of Rubus idaeus cv. Nova: Transcriptome sequencing and de novo assembly for subsequent functional genomics approaches. Plant Cell Rep. 2014, 33, 1617–1628.
- Hyun, T.K.; Lee, S.; Rim, Y.; Kumar, R.; Han, X.; Lee, S.Y.; Lee, C.H.; Kim, J.Y. de-novo RNA Sequencing and Metabolite Profiling to Identify Genes Involved in Anthocyanin Biosynthesis in Korean Black Raspberry (Rubus coreanus Miquel). PLoS ONE 2014, 9, e88292.
- Garcia-Seco, D.; Zhang, Y.; Gutierrez-Mañero, F.; Martin, C.; Ramos-Solano, B. RNA-Seq analysis and transcriptome assembly for blackberry (Rubus sp. Var. Lochness) fruit. BMC Genom. 2015, 16, 5.
- Choquer, M.; Fournier, E.; Kunz, C.; Levis, C.; Pradier, J.M.; Simon, A.; Viaud, M. Botrytis cinerea virulence factors: New insights into a necrotrophic and polyphageous pathogen. FEMS Microbiol. Lett. 2007, 277, 1–10.
- González, G.; Fuentes, L.; Moya-León, M.; Sandoval, C.; Herrera, R. Characterization of two PR genes from Fragaria chiloensis in response to Botrytis cinerea infection: A comparison with F. x annanassa. Physiol. Mol. Plant Pathol. 2013, 82, 73–80.
- Kozhar, O.; Peever, T.L. How Does Botrytis cinerea Infect Red Raspberry? Phytopathology 2018, 108, 1287–1298.
- Stewart, D.; Iannetta, P.P.; Davies, H.V. Ripening-related changes in raspberry cell wall composition and structure. Phytochemistry 2001, 56, 423–428.
- Figueroa, C.R.; Rosli, H.G.; Civello, P.M.; Martínez, G.A.; Herrera, R.; Moya-León, M.A. Changes in cell wall polysaccharides and cell wall degrading enzymes during ripening of Fragaria chiloensis and Fragaria × ananassa fruits. Sci. Hortic. 2010, 124, 454–462.
- Kader, A.A. Flavor quality of fruits and vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868.
- Aprea, E.; Biasioli, F.; Gasperi, F. Volatile compounds of raspberry fruit: From analytical methods to biological role and sensory impact. Molecules 2015, 20, 2445–2474.
- Yan, J.W.; Ban, Z.J.; Lu, H.Y.; Li, D.; Poverenov, E.; Luo, Z.S.; Li, L. The aroma volatile repertoire in strawberry fruit: A review. J. Sci. Food Agric. 2018, 98, 4395–4402.
- Ulrich, D.; Kecke, S.; Olbricht, K. What Do We Know about the Chemistry of Strawberry Aroma? J. Agric. Food Chem. 2018, 66, 3291–3301.
- El Hadi, M.A.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229.
- Kim, S.; Park, M.; Yeom, S.I.; Kim, Y.M.; Lee, J.M.; Lee, H.A.; Seo, E.; Choi, J.; Cheong, K.; Kim, K.T. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat. Genet. 2014, 46, 270–278.
- Daminato, M.; Guzzo, F.; Casadoro, G. A SHATTERPROOF-like gene controls ripening in non-climacteric strawberries, and auxin and abscisic acid antagonistically affect its expression. J. Exp. Bot. 2013, 64, 3775–3786.
- Chervin, C.; El-Kereamy, A.; Roustan, J.-P.; Latché, A.; Lamon, J.; Bouzayen, M. Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Sci. 2004, 167, 1301–1305.
- Böttcher, C.; Keyzers, R.A.; Boss, P.K.; Davies, C. Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3-1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J. Exp. Bot. 2010, 61, 3615–3625.
- Jiang, Y.; Joyce, D.C. ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul. 2003, 39, 171–174.
- Gillaspy, G.; Ben-David, H.; Gruissem, W. Fruits: A developmental perspective. Plant Cell 1993, 5, 1439–1451.
- De Jong, M.; Wolters-Arts, M.; Feron, R.; Mariani, C.; Vriezen, W.H. The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J. 2009, 57, 160–170.
- Dorcey, E.; Urbez, C.; Blazquez, M.A.; Carbonell, J.; Perez-Amador, M.A. Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J. 2009, 58, 318–332.
- Mariotti, L.; Picciarelli, P.; Lombardi, L.; Ceccarelli, N. Fruit-set and early fruit growth in tomato are associated with increases in indoleacetic acid, cytokinin, and bioactive gibberellin contents. J. Plant Growth Regul. 2011, 30, 405–415.
- Carrera, E.; Ruiz-Rivero, O.; Peres, L.E.; Atares, A.; Garcia-Martinez, J.L. Characterization of the procera tomato mutant shows novel functions of the SlDELLA protein in the control of flower morphology, cell division and expansion, and the auxin-signaling pathway during fruit-set and development. Plant Physiol. 2012, 160, 1581–1596.
- Parada, F.; Espinoza, C.; Arce-Johnson, P. Chapter 7: Phytohormonal Control over the Grapevine Berry Development. In Phytohormones—Signaling Mechanisms and Crosstalk in Plant Development and Stress Responses; InTech.: Acton, MA, USA, 2017; pp. 143–159.
- Garrido-Bigotes, A.; Figueroa, P.M.; Figueroa, C.R. Jasmonate Metabolism and Its Relationship with Abscisic Acid during Strawberry Fruit Development and Ripening. J. Plant Growth Regul. 2018, 37, 101–113.
- Bernales, M.; Monsalve, L.; Ayala-Raso, A.; Valdenegro, M.; Martínez, J.P.; Travisany, D.; Defilippi, B.; González-Agüero, M.; Cherian, S.; Fuentes, L. Expression of two indole-3-acetic acid (IAA)-amido synthetases (GH3) genes during fruit development and ripening of raspberry (Rubus idaeus Heritage). Sci. Hortic. 2019, 246, 168–175.
- Travisany, D.; Ayala-Raso, A.; Di Genova, A.; Monsalve, L.; Bernales, M.; Martínez, J.P.; González-Agüero, M.; Defilippi, B.; Cherian, S.; Maass, A.; et al. RNA-Seq analysis and transcriptome assembly of raspberry fruit (Rubus idaeus ¨Heritage¨) revealed several candidate genes involved in fruit development and ripening. Sci. Hort. 2019, 254, 26–34.
- Horvath, D.P.; Anderson, J.V.; Chao, W.S.; Foley, M.E. Knowing when to grow: Signals regulating bud dormancy. Trends Plant Sci. 2003, 8, 534–540.
- Gray, W.M. Hormonal regulation of plant growth and development. PLoS Biol. 2004, 2, E311.
- Liu, K.; Kang, B.C.; Jiang, H.; Moore, S.L.; Li, H.; Watkins, C.B.; Setter, T.L.; Jahn, M.M. A GH3-like gene, CcGH3, isolated from Capsicum chinense L. fruit is regulated by auxin and ethylene. Plant Mol. Biol. 2005, 58, 447–464.
- Kumar, R.; Agarwal, P.; Tyagi, A.K.; Sharma, A.K. Genome-wide investigation and expression analysis suggest diverse roles of auxin responsive GH3 genes during development and response to different stimuli in tomato (Solanum lycopersicum). Mol. Genet. Genom. 2012, 287, 221–235.
- Kumar, R.; Sharma, M.K.; Kapoor, S.; Tyagi, A.K.; Sharma, A.K. Transcriptome analysis of rin mutant fruit and in silico analysis of promoters of differentially regulated genes provides insight into LeMADSRIN-regulated ethylene-dependent as well as ethylene-independent aspects of ripening in tomato. Mol. Genet. Genom. 2012, 287, 189–203.
- Jiang, Y.; Joyce, D.C.; Macnish, A.J. Effect of abscisic acid on banana fruit ripening in relation to the role of ethylene. J. Plant Growth Regul. 2000, 19, 106–111.
- Gambetta, G.A.; Matthews, M.A.; Shaghasi, T.H.; McElrone, A.J.; Castellarin, S.D. Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape. Planta 2010, 232, 219–234.
- Zhang, M.; Yuan, B.; Leng, P. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J. Exp. Bot. 2009, 60, 1579–1588.
- Sun, L.; Sun, Y.F.; Zhang, M.; Wang, L.; Ren, J.; Cui, M.; Wang, Y.; Ji, K.; Li, P.; Li, Q.; et al. Suppression of 9-cis-epoxycarotenoid dioxygenase, which encodes a key enzyme in abscisic acid biosynthesis, alters fruit texture in transgenic tomatoes. Plant Physiol. 2012, 158, 283–298.
- Sun, L.; Yuan, B.; Zhang, M.; Wang, L.; Cui, M.M.; Wang, Q.; Leng, P. Fruit-specific RNAi-mediated suppression of SlNCED1 increases both lycopene and β-carotene contents in tomato fruit. J. Exp. Bot. 2012, 63, 3097–3108.
- Ziliotto, F.; Corso, M.; Rizzini, F.M.; Rasori, A.; Botton, A.; Bonghi, C. Grape berry ripening delay induced by a pre-véraison NAA treatment is paralleled by a shift in the expression pattern of auxin- and ethylene-related genes. BMC Plant Biol. 2012, 12, 185.
- Wong, D.C.; Lopez Gutierrez, R.; Dimopoulos, N.; Gambetta, G.A.; Castellarin, S.D. Combined physiological, transcriptome, and cis-regulatory element analyses indicate that key aspects of ripening, metabolism, and transcriptional program in grapes (Vitis vinifera L.) are differentially modulated accordingly to fruit size. BMC Genom. 2016, 17, 416.
- Medina-Puche, L.; Blanco-Portales, R.; Molina-Hidalgo, F.J.; Cumplido-Laso, G.; García-Caparrós, N.; Moyano-Cañete, E.; Caballero-Repullo, J.L.; Muñoz-Blanco, J.; Rodríguez-Franco, A. Extensive transcriptomic studies on the roles played by abscisic acid and auxins in the development and ripening of strawberry fruits. Funct. Integr. Genom. 2016, 16, 671–692.
- Böttcher, C.; Burbidge, C.A.; Boss, P.K.; Davies, C. Interactions between ethylene and auxin are crucial to the control of grape (Vitis vinifera L.) berry ripening. BMC Plant Biol. 2013, 13, 222.
- Jung, C.J.; Hur, Y.Y.; Yu, H.J.; Noh, J.H.; Park, K.S.; Lee, H.J. Gibberellin application at prebloom in grapevines down-regulates the expressions of VvIAA9 and VvARF7, negative regulators of fruit set initiation, during parthenocarpic fruit development. PLoS ONE 2014, 9, e95634.
- Lu, L.; Liang, J.; Zhu, X.; Xiao, K.; Li, T.; Hu, J. Auxin- and cytokinin-induced berries set in grapevine partly rely on enhanced gibberellin biosynthesis. Tree Genet. Genomes 2016, 12, 41.
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signalling. Annu. Rev. Plant Biol. 2010, 61, 593–620.
- Kudla, J.; Batistic, O.; Hashimoto, K. Calcium signals: The lead currency of plant information processing. Plant Cell 2010, 22, 541–563.
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit Calcium: Transport and Physiology. Front. Plant Sci. 2016, 7, 569.
- Martins, V.; Garcia, A.; Costa, C.; Sottomayor, M.; Gerós, H. Calcium- and hormone-driven regulation of secondary metabolism and cell wall enzymes in grape berry cells. J. Plant Physiol. 2018, 231, 57–67.
- Hardtke, C.S. Transcriptional auxin-brassinosteroid crosstalk: who’s talking? Bioessays 2007, 29, 1115–1123.
- De Jong, M.; Wolters-Arts, M.; Garcia-Martinez, J.L.; Mariani, C.; Vriezen, W.H. The Solanum lycopersicum AUXIN RESPONSE FACTOR 7 (SlARF7) mediates cross-talk between auxin and gibberellin signalling during tomato fruit set and development. J. Exp. Bot. 2011, 62, 617–626.
- Medina-Puche, L.; Cumplido-Laso, G.; Amil-Ruiz, F.; Hoffmann, T.; Ring, L.; Rodríguez-Franco, A.; Caballero, J.L.; Schwab, W.; Muñoz-Blanco, J.; Blanco-Portales, R. MYB10 plays a major role in the regulation of flavonoid/phenylpropanoid metabolism during ripening of Fragaria x ananassa fruits. J. Exp. Bot. 2014, 65, 401–417.
- Chen, J.; Mao, L.; Lu, W.; Ying, T.; Luo, Z. Transcriptome profiling of postharvest strawberry fruit in response to exogenous auxin and abscisic acid. Planta 2016, 243, 183–197.
- Figueroa, C.R.; Opazo, M.C.; Vera, P.; Arriagada, O.; Díaz, M.; Moya-León, M.A. Effect of postharvest treatment of calcium and auxin on cell wall composition and expression of cell wall-modifying genes in the Chilean strawberry (Fragaria chiloensis) fruit. Food Chem. 2012, 132, 2014–2022.
- Liao, X.; Li, M.; Liu, B.; Yan, M.; Yu, X.; Zi, H.; Liu, R.; Yamamuro, C. Interlinked regulatory loops of ABA catabolism and biosynthesis coordinate fruit growth and ripening in woodland strawberry. Proc. Natl. Acad. Sci. USA 2018, 115, E11542–E11550.
- Mukkun, L.; Singh, Z. Methyl jasmonate plays a role in fruit ripening of ‘Pajaro’ strawberry through stimulation of ethylene biosynthesis. Sci. Hortic. 2009, 123, 5–10.
- Concha, C.M.; Figueroa, N.E.; Poblete, L.A.; Oñate, F.A.; Schwab, W.; Figueroa, C.R. Methyl jasmonate treatment induces changes in fruit ripening by modifying the expression of several ripening genes in Fragaria chiloensis fruit. Plant Physiol. Biochem. 2013, 70, 433–444.
- Li, T.; Xu, Y.; Zhang, L.; Ji, Y.; Tan, D.; Yuan, H.; Wang, A. The jasmonate-activated transcription factor MdMYC2 regulates ETHYLENE RESPONSE FACTOR and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell 2017, 29, 1316–1334.
- Moro, L.; Hassimotto, N.M.A.; Purgatto, E. Postharvest Auxin and Methyl Jasmonate Effect on Anthocyanin Biosynthesis in Red Raspberry (Rubus idaeus L.). J. Plant Growth Regul. 2017, 36, 773.
- Yuan, H.Y.; Chen, L.G.; Paliyath, G.; Sullivan, A.; Murr, D.P. Characterization of microsomal and mitochondrial phospholipase D activities and cloning of a phospholipase D alpha cDNA from strawberry fruits. Plant Physiol. Biochem. 2005, 43, 535–547.
- El Kayal, W.E.; Paliyath, G.; Sullivan, J.A.; Subramanian, J. Phospholipase D inhibition by hexanal is associated with calcium signal transduction events in raspberry. Hortic. Res. 2017, 4, 17042.
More
