Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Tanmay Sarkar and Version 2 by Camila Xu.
Quorum sensing (QS) is a density dependent cell-to-cell communication mechanism responsible for controlling pathogenicity with the regulation of gene expression.
- ESKAPE pathogens
- quorum sensing
- QS inhibitors
1. Introduction
Over the years various, selective pressures on pathogenic bacteria have resulted in the employment of different ways to adapt various types of environmental nooks. One such adaptive approach is formation of biofilm, which is a consortia of bacterial cells that remain embedded within a self-secreted extracellular polymeric substance (EPS) helping in the process of surface attachment [1][2][3][1,2,3]. QS plays a pivotal role in the development of biofilm [4]. The sessile microcolonies that are associated with the biofilm structure portray an elevated level of adaptive resistance to antibiotics or antibacterial drugs in comparison to planktonic counterparts [5]. Adaptive resistance against antibiotics acts as a global threat in the treatment of biofilm-associated acute and chronic infections such as surgical wound infections, nosocomial pneumonia, infections in wounds due to burns, uro-catheter infections and pneumonia caused due to ventilation [6]. Thus, formation of biofilms is actually responsible for causing various problems in food industries, health-care sectors and other medical fields [7][8][7,8]. Amongst them, the ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter sp.) bacteria are accountable for a majority of the nosocomial diseases and are capable of evasion of the biocidal action of antimicrobial agents. For this very reason, the overuse and misuse of antibacterial drugs led the ESKAPE bacteria to easily ‘escape’ the actions of these drugs and cause different chronic diseases, which are not easily cured. So, alternative ways to treat and cure these diseases caused by ESKAPE pathogens are of great importance to safeguard human health. Such looming health risk has restimulated the surge for the examination of new sources of anti-biofilm agents with utmost priority [9]; and therefore, several natural resources are explored, which include different phytocompounds and microbial metabolites. Until now, many natural compounds have been isolated and identified possessing antibacterial attributes [10], of which a number of plant compounds are identified.
Phytocompounds are preferred for their relatively non-toxic nature, biocompatibility, easy availability, biodegradability, and eco-friendliness [11]. Different plant parts of Teucrium polium [12], Thymus musilii Velen [13], Carum copticum Carum copticum [14][14], Salvadora persica [15], and Syzygium jambos [16] are found to have antibacterial activities. Pteridophytes such as Tectaria coadunata [17], different species of the genus Selaginella [18], and Adiantum philippense [19] are noteworthy for their therapeutic potential. Some members of gymnosperms, namely Pinus strobus, and Pinus pinastar are known for their antimicrobial phytocompounds [20]. Moreover, many members of the plant family Lamiaceae are found to have medicinal properties [21].
Although many of the abovementioned herbal sources with antimicrobial activities do not show anti-biofilm efficacies, precisely targeting QS signals, bioactive compounds obtained from plants such as Taraxacum, Tussilago and Scutellera have been reported to show an anti-QS effect against biofilm formation in ESKAPE microbes [22]. These QS inhibiting phytocompounds either disrupt the pathways or inhibit the genes responsible for QS in biofilm forming bacteria including the ESKAPE. To find out some suitable alternative therapeutic measures, a thorough insight is required to discuss the anti-biofilm activities of the phytocompounds, their strategies to disrupt quorum sensing ability to address the nosocomial disease-causing deadly organisms, which may be effectively used to combat and cure chronic biofilm-related infections and there lies the rationale of the present review, whereby we intend to focus on the mechanisms by which various natural compounds can inhibit QS, thereby regulating biofilm formation and their effectiveness against ESKAPE bacteria. Such newly identified natural compounds are considered as promising therapeutic agents to combat and cure chronic biofilm-related infections.
2. ESKAPE Pathogens: A Global Menace
Antimicrobial resistance (AMR) is a global threat to public health. This AMR is acquired by bacteria through horizontal gene transfer (HGT) via chromosomes, genetic mutations, transposons, plasmids, and some other mobile genetic elements [5][23][5,23]. The first-rate class of opportunistic pathogens that are considered as a universal threat to mankind are known as ESKAPE, that includes Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumonia, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter sp. Thus, it can be said that AMR can be found in both Gram-negative and Gram-positive bacterial strains. The rate at which AMR is being developed in these pathogens is alarmingly increasing. A. baumannii, K. pneumonia, and P. aeruginosa are resistant to carbapenem, and members of Enterobacteriaceae and S. aureus are partially resistant to vancomycin and fully resistant to methicillin and thus they are called methicillin resistant S. aureus (MRSA). All these resistant bacterial classes are also known as superbugs. Such pathogens are known to cause lethal diseases among immunocompromised and severely ill individuals because of improper treatment [24]. Hence, the effects of ESKAPE are devastating.
2.1. Enterococcus faecium
E. faecium is a spherical (coccus) Gram-positive bacterium occurring in chains/pairs and is generally responsible for causing nosocomial septicemia amongst immunocompromised patients. βlactam antibiotics such as penicillin and different last resource antibiotics are ineffective against E. faecium. Resistance to vancomycin, has in particular led to the enhanced development of Enterococci strains with vancomycin resistance. A gene in vancomycin resistant Enterococci (VRE) codes for a putative Enterococcal surface protein helping to form more thick biofilms [25]. This leads to the development of infections such as bacteremia, urinary tract infections (UTI), endocarditis, and intra-abdominal infections. Enterococcal bacteria are now considered the third most frequent nosocomial pathogen causing 14% of hospital-acquired contaminations in the United States (US) from the year 2011 to 2014, and apart from nosocomial infections, are also known to cause community-acquired endocarditis [25].
2.2. Staphylococcus aureus
S. aureus is a spherical (coccal) Gram-positive bacterium generally found in the skin microbiota of humans and is mostly harmful amongst immunocompromised individuals. Since it is normally present within the skin microflora of humans, it can cause infections only by penetrating cuts, wounds, or bruises, which are regions not inhabited by it. S. aureus is responsible for causing medical implant infections by formation of biofilms, which are not easily cleared by treatment with antibiotics. Around 25% of strains of S. aureus can secrete the exotoxin TSST-1 causing toxic shock syndrome. MRSA are the largest group of S. aureus to have developed resistance to β-lactam antibiotics. Resistance against β-lactam antibiotics are acquired by the expression of mecA encoding a protein with low affinity for binding to penicillin [26]. Thus, they are known to cause many healthcare associated infections such as infections in prosthetic devices and in infective endocarditis. Pathogenic and β-lactam resistant strains are a few of the major causative agents of soft tissue and skin infections. Many strains have also gained resistance to vancomycin, due to its overuse in treating S. aureus infections [27].
2.3. Klebsiella pneumoniae
K. pneumoniae is a rod-shaped bacillus Gram-negative bacterium. One third amount of all infections caused by gram-negative bacteria is due to this microbe. They are responsible in causing septicemia, UTI, cystitis, infections in surgical wounds, endocarditis, and pneumonia. It also causes pyogenic liver abscess, necrotizing pneumonia and endogenous endophthalmitis. Infections caused due to K. pneumoniae leads to higher mortality rates, prolonged period of hospitalization and treatments of exorbitant rates. Strains that are carbapenem resistant K. pneumoniae (CRKP) are known to develop β-lactamases making them resistant towards usually used antibiotics such as carbapenem [28].
2.4. Acinetobacter baumannii
A. baumannii is a purely aerobic, Gram-negative, non-fastidious, non-fermenting, oxidase-negative, catalase-positive coccobacillus (pleomorphic) bacterium known to cause almost 2% of all nosocomial infections. 45% of the strains of this lethal opportunistic pathogen have been found to be multidrug resistant (MDR) [29]. The most caused infections by it are pneumonia associated with ventilation and central-line associated infections in bloodstream. Because of their ability of biofilm formation, desiccation resistance and existence of major pathogenic properties such as glycoconjugates, surface adhesions and their system of secretion help A. baumannii to survive under extreme unfavorable conditions [29].
2.5. Pseudomonas aeruginosa
P. aeruginosa is a Gram-negative gamma-proteobacterium having a very slightly permeable outer membrane and multi-transport systems providing it an innate resistance to several antibiotics. It can employ diverse mechanisms such as efflux pumps, porin channel alteration, β-lactamases and modifications of the target for developing resistance towards antimicrobial substances. Patients suffering from cystic fibrosis (CF) are at a higher risk of developing this infection due to its capacity of biofilm formation and persistent cells inside the lungs [30]. P. aeruginosa is immensely resistant to fluoroquinolones due to the point mutations on topoisomerase IV or DNA gyrase.
2.6. Enterobacter sp.
Enterobacter sp. are facultatively anaerobic, rod-shaped, Gram-negative bacteria and belong to the family of Enterobacteriaceae. Patients who are immunocompromised or implanted with implantable medical device (IMD), or on support of mechanical ventilation, are mostly prone to acquire respiratory or urinary tract infections caused by this pathogen. Enterobacter cloacae is the commonly responsible pathogen for causing 4–5% of nosocomial pneumonia, bacteremia and UTIs [31]. They are resistant towards a broad spectrum of antibiotics such as carbapenems via extended spectrum β-lactamases (ESBLs) encoded by plasmids.
3. Mechanism of QS
The mechanism of QS relies on the biosynthesis, secretion, and assimilation of autoinducers (AIs) in the circumambient medium, the concentration of which is dependent on the population density of the bacterial cells. Extracellular signaling molecules and AIs accumulated within the circumambient medium in proportion to the cellular density and thus they are used for intercellular communication [32]. Their role involves the regulation of gene expression of the cells present in the community, thereby regulating a variety of responses of bacterial cells. Different physiological processes of bacteria such as motility, pathogenicity, formation of biofilms, luminescence, genetic competence development, sporulation, generation and secretion of proteolytic enzymes, biosynthesis of peptide antibiotics, and fluorescence are controlled by QS. Researchers have found that the production of signal molecules is dependent on the mechanism of autoinducing and this type of mechanism is different in Gram-positive and Gram-negative bacteria [33]. Such signaling molecules, along with their receptors, have been classified into three major groups: (i) autoinducer (AI)-2 in Gram-negative as well as in Gram-positive bacteria for interspecies communication, (ii) autoinducing peptides or oligopeptides comprising 5–34 residues of amino acids that usually take part in intercellular communication among gram-positive bacteria; a majority of these peptides are effluxed by specific systems and are modified post-translationally in different ways so that they are sensed by other cells via membrane-embedded receptors, which are part of 2-component regulatory system and (iii) N-acyl homoserine lactones (AHLs) that differ in length and state of oxidation of the acyl side chain and are secreted by Gram-negative bacteria in order to monitor their density of population in QS-mediated gene expression control and the signals produced by the members of the LuxI protein family (Figure 1).
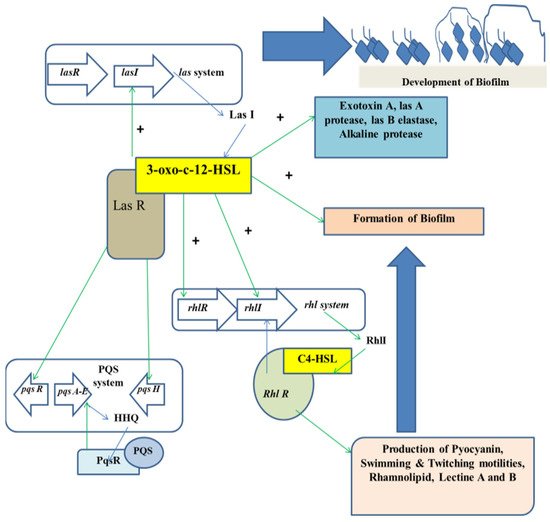

Figure 1.
Regulation of QS in the development of biofilm.
3.1. QS Mediated by AI-2
This system of QS-mediated biofilm formation is found in both Gram-positive and Gram-negative bacteria. AI-2 is also known as furanosyl borate diester. This molecule has the capacity to switch between intra-and inter-species signalling on the basis of density and concentration at threshold [34]. AI-2 is chemically called a furanosylborate diester that is produced by the members of LuxS protein family [35]. In Gram-positive bacteria, the precursor peptide molecules of AI are altered and exported out of the cell via ATP-binding cassette (ABC) exporter complex. As the peptide AI concentration reaches the threshold value, the protein in the sensor kinase will be activated and carry out phosphorylation of the protein regulating the response, and this phosphorylated protein will bind to the target promoter leading to gene regulation by QS. Contrary to this, in Gram-negative bacteria, AIs are secreted and freely diffused out of the cell. Once the AI concentration reaches the threshold value, a positive loop of feedback is generated, causing more biosynthesis of AIs. As the AI concentration enhances in proportion to the increase in bacterial population, then after reaching a specific point, these molecules again diffuse in the bacteria for regulating specific gene transcription triggering the production and secretion of pathogenic factors, antibiotics, and form biofilms.
3.2. QS Mediated by Secretion of Peptide
The molecules responsible for QS in Gram-positive bacteria are based on the biosynthesis of oligopeptide from the peptide. These molecules are known as auto-inducing oligopeptides (AIPs), which are utilized as signaling molecules in QS sensing. Such AIPs are produced into the extracellular environment and they possess an affinity of binding to the histidine (His) membrane receptor kinase [36]. The AIP molecular expression is dependent on the gene family of agrD. After the AIPs are expressed, the conformation of the agrB protein bound to the membrane is altered by the incorporation of thiolactone and so they may be effluxed to the circumambient media in the form of oligopeptide. As the extracellular AIP concentration attains threshold, these molecules bind to the agrC receptor (membrane bound receptor kinase). The transmembrane transduction of signals produces the auto-phosphorylated AgrA, thereby triggering various signaling pathways bringing about the expression of agrBDCA protein. This system activation is based on the concentration of cell density. As the density of the cell increases, the system of agr will activate, thereby switching the physiological states of bacteria on the proteases and toxin production causing adherence, commensalism, aggression, and invasion [37].
3.3. QS Mediated by AHLs
In case of Gram-negative bacteria such as P. aeruginosa, RhlR-I and LasR-I systems take part in the QS regulation. The AHL molecule biosynthesis is carried out by a certain enzyme known as lactone synthase or LuxI. This enzyme is secreted within the extracellular medium. Once the cell density is increased or attained quorum, the AHL concentration in the extracellular medium reaches a critical concentration in this stage and the AHLs diffuse into the cells, thereby interacting with the transcriptional regulators [38]. In P. aeruginosa, AHL molecules are produced in two different forms, which are C4-HSL and 3OC12-HSL. Each form of AHL molecule binds to their respective transcriptional regulators, that is RhlR to C4-HSL, and LasR to 3OC12-HSL, respectively. The complexes thus formed activate several transcriptional regulators such as toxA, LasB, and LasI. The family gene regulator activation is also dependent on the concentration of the AHL molecules.
Alteration of physiological processes is controlled through AHLs inducing the expression of the QS genes [39]. Thus, all the AHLs found so far consist of an acyl chain having an even number of atoms of carbon ranging from 4–14 in terms of length and attached to the homoserine lactone moiety [40]. The constituents of the AHL regulated QS system belong to the LuxI and LuxR protein families. LuxI is responsible for the generation of AHLs, whereas LuxR is known to repress or activate certain gene transcription such as those of the virulent genes.
3.4. QS Mediated by Other Systems
Sometimes, a natural modification in the system of QS may be observed. There exist some strains of bacteria that do not synthesize self-induced molecules or peptides, while they respond with respect to the auto-induced signals produced by other bacteria. For example, E. coli contains a signaling molecule known as SdiA (a molecule which is homologous to LuxR) in response to the corresponding signals produced by other bacteria. Apart from this, Burkholderia cepacia responds to the QS signals of P. aeruginosa through CF signal molecules [41].
