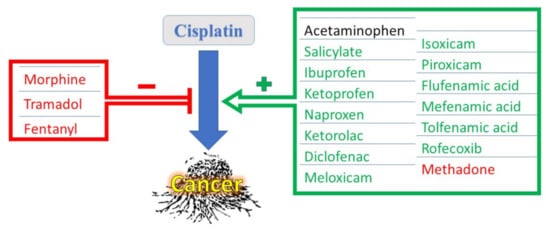
Cisplatin (CDDP), one of the most eminent cancer chemotherapeutic agents, has been successfully used to treat more than half of all known cancers worldwide. Despite its effectiveness, CDDP might cause severe toxic adverse effects on multiple body organs during cancer chemotherapy, including the kidneys, heart, liver, gastrointestinal tract, and auditory system, as well as peripheral nerves causing severely painful neuropathy. The latter, among other pains patients feel during chemotherapy, is an indication for the use of analgesics during treatment with CDDP. Different types of analgesics, such as acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDS), and narcotic analgesics, could be used according to the severity of pain. Administered analgesics might modulate CDDP’s efficacy as an anticancer drug. NSAIDS, on one hand, might have cytotoxic effects on their own and few of them can potentiate CDDP’s anticancer effects via inhibiting the CDDP-induced cyclooxygenase (COX) enzyme, or through COX-independent mechanisms. On the other hand, some narcotic analgesics might ameliorate CDDP’s anti-neoplastic effects, causing chemotherapy to fail. Concerning safety, some analgesics share the same adverse effects on normal tissues as CDDP, augmenting its potentially hazardous effects on organ impairment.
- cisplatin
- analgesics
- acetaminophen
- non-steroidal anti-inflammatory drugs
- morphine
- cytotoxicity
1. Interactions of NSAIDs with CDDP
1.1. Interactions of Salicylates with CDDP
1.2. Interaction of Propionic Acid-Derived NSAIDs (Profens) with CDDP
1.3. Interaction of Acetic Acid-Derived NSAIDS with CDDP
1.4. Interaction of Enolic Acid Derivatives of NSAIDs (Oxicams) with CDDP
1.5. Interaction of Anthranilic Acid and Naphthylalanine Derivatives of NSAIDs (Fenamates) with CDDP
1.6. Interaction of COX-II Selective NSAIDS (Coxibs) with CDDP
References
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs); StatPearls Publishing: Treasure Island, FL, USA, 2021; pp. 1–11.
- Ibrahim, M.A.; Albahlol, I.A.; Wani, F.A.; Tammam, A.A.-E.; Kelleni, M.T.; Sayeed, M.U.; El-Fadeal, N.M.A.; Mohamed, A.A. Resveratrol protects against cisplatin-induced ovarian and uterine toxicity in female rats by attenuating oxidative stress, inflammation and apoptosis. Chem. Interact. 2021, 338, 109402.
- Xing, L.; Yang, C.-X.; Zhao, D.; Shen, L.-J.; Zhou, T.-J.; Bi, Y.-Y.; Huang, Z.-J.; Wei, Q.; Li, L.; Li, F.; et al. A carrier-free anti-inflammatory platinum (II) self-delivered nanoprodrug for enhanced breast cancer therapy. J. Control. Release 2021, 331, 460–471.
- Dehkordi, N.G.; Mirzaei, S.A.; Elahian, F. Pharmacodynamic mechanisms of anti-inflammatory drugs on the chemosensitization of multidrug-resistant cancers and the pharmacogenetics effectiveness. Inflammopharmacology 2021, 29, 49–74.
- Ravera, M.; Zanellato, I.; Gabano, E.; Perin, E.; Rangone, B.; Coppola, M.; Osella, D. Antiproliferative Activity of Pt(IV) Conjugates Containing the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Ketoprofen and Naproxen. Int. J. Mol. Sci. 2019, 20, 3074.
- O’Brien, A.J.; Villani, L.A.; Broadfield, L.A.; Houde, V.P.; Galic, S.; Blandino, G.; Kemp, B.E.; Tsakiridis, T.; Muti, P.; Steinberg, G.R. Salicylate activates AMPK and synergizes with metformin to reduce the survival of prostate and lung cancer cells ex vivo through inhibition of de novo lipogenesis. Biochem. J. 2015, 469, 177–187.
- Broadfield, L.A.; Marcinko, K.; Tsakiridis, E.; Zacharidis, P.G.; Villani, L.; Lally, J.S.V.; Menjolian, G.; Maharaj, D.; Mathurin, T.; Smoke, M.; et al. Salicylate enhances the response of prostate cancer to radiotherapy. Prostate 2019, 79, 489–497.
- Karalis, T.T.; Chatzopoulos, A.; Kondyli, A.; Aletras, A.J.; Karamanos, N.K.; Heldin, P.; Skandalis, S.S. Salicylate suppresses the oncogenic hyaluronan network in metastatic breast cancer cells. Matrix Biol. Plus 2020, 6, 100031.
- Kumar, A.; Singh, S.M. Priming effect of aspirin for tumor cells to augment cytotoxic action of cisplatin against tumor cells: Implication of altered constitution of tumor microenvironment, expression of cell cycle, apoptosis, and survival regulatory molecules. Mol. Cell. Biochem. 2012, 371, 43–54.
- Liao, D.; Zhong, L.; Duan, T.; Zhang, R.-H.; Wang, X.; Wang, G.; Hu, K.; Lv, X.; Kang, T. Aspirin Suppresses the Growth and Metastasis of Osteosarcoma through the NF-κB Pathway. Clin. Cancer Res. 2015, 21, 5349–5359.
- Khan, P.; Bhattacharya, A.; Sengupta, D.; Banerjee, S.; Adhikary, A.; Das, T. Aspirin enhances cisplatin sensitivity of resistant non-small cell lung carcinoma stem-like cells by targeting mTOR-Akt axis to repress migration. Sci. Rep. 2019, 9, 16913.
- Jiang, W.; Yan, Y.; Chen, M.; Luo, G.; Hao, J.; Pan, J.; Hu, S.; Guo, P.; Li, W.; Wang, R.; et al. Aspirin enhances the sensitivity of colon cancer cells to cisplatin by abrogating the binding of NF-κB to the COX-2 promoter. Aging 2020, 12, 611–627.
- Zhao, M.; Wang, T.; Hui, Z. Aspirin overcomes cisplatin resistance in lung cancer by inhibiting cancer cell stemness. Thorac. Cancer 2020, 11, 3117–3125.
- Guo, J.; Zhu, Y.; Yu, L.; Li, Y.; Guo, J.; Cai, J.; Liu, L.; Wang, Z. Aspirin inhibits tumor progression and enhances cisplatin sensitivity in epithelial ovarian cancer. PeerJ 2021, 9, e11591.
- Zou, Z.; Zheng, W.; Fan, H.; Deng, G.; Lu, S.-H.; Jiang, W.; Yu, X. Aspirin enhances the therapeutic efficacy of cisplatin in oesophageal squamous cell carcinoma by inhibition of putative cancer stem cells. Br. J. Cancer 2021, 125, 1–13.
- Cheng, Q.; Shi, H.; Wang, H.; Min, Y.; Wang, J.; Liu, Y. The ligation of aspirin to cisplatin demonstrates significant synergistic effects on tumor cells. Chem. Commun. 2014, 50, 7427–7430.
- Pathak, R.K.; Marrache, S.; Choi, J.H.; Berding, T.B.; Dhar, S. The Prodrug Platin-A: Simultaneous Release of Cisplatin and Aspirin. Angew. Chem. Int. Ed. 2014, 53, 1963–1967.
- Ulubaş, B.; Cimen, M.Y.B.; Apa, D.D.; Saritaş, E.; Muslu, N.; Cimen, Ö.B. The Protective Effects of Acetylsalicylic Acid on Free Radical Production in Cisplatin Induced Nephrotoxicity: An Experimental Rat Model. Drug Chem. Toxicol. 2003, 26, 259–270.
- Yıldırım, M.; Inançlı, H.M.; Samancı, B.; Oktay, M.F.; Enöz, M.; Topçu, I. Preventing cisplatin induced ototoxicity by N-acetylcysteine and salicylate. Kulak Burun Bogaz Ihtis Derg 2010, 20, 173–183.
- Cetin, D.; Hacımuftuoglu, A.; Tatar, A.; Turkez, H.; Togar, B. The in vitro protective effect of salicylic acid against paclitaxel and cisplatin-induced neurotoxicity. Cytotechnology 2016, 68, 1361–1367.
- Kłobucki, M.; Urbaniak, A.; Grudniewska, A.; Kocbach, B.; Maciejewska, G.; Kiełbowicz, G.; Ugorski, M.; Wawrzeńczyk, C. Syntheses and cytotoxicity of phosphatidylcholines containing ibuprofen or naproxen moieties. Sci. Rep. 2019, 9, 220.
- Alves, S.R.; Colquhoun, A.; Wu, X.Y.; Silva, D.D.O. Synthesis of terpolymer-lipid encapsulated diruthenium(II, III)-anti-inflammatory metallodrug nanoparticles to enhance activity against glioblastoma cancer cells. J. Inorg. Biochem. 2020, 205, 110984.
- Endo, H.; Yano, M.; Okumura, Y.; Kido, H. Ibuprofen enhances the anticancer activity of cisplatin in lung cancer cells by inhibiting the heat shock protein 70. Cell Death Dis. 2014, 5, e1027.
- Petruzzella, E.; Sirota, R.; Solazzo, I.; Gandin, V.; Gibson, D. Triple action Pt(iv) derivatives of cisplatin: A new class of potent anticancer agents that overcome resistance. Chem. Sci. 2018, 9, 4299–4307.
- Fan, C.-C.; Tsai, S.-T.; Lin, C.-Y.; Chang, L.-C.; Yang, J.-C.; Chen, G.; Sher, Y.-P.; Wang, S.-C.; Hsiao, M.; Chang, W. EFHD2 contributes to non-small cell lung cancer cisplatin resistance by the activation of NOX4-ROS-ABCC1 axis. Redox Biol. 2020, 34, 101571.
- Gunjal, P.M.; Schneider, G.; Ismail, A.A.; Kakar, S.S.; Kucia, M.; Ratajczak, M.Z. Evidence for induction of a tumor metastasis-receptive microenvironment for ovarian cancer cells in bone marrow and other organs as an unwanted and underestimated side effect of chemotherapy/radiotherapy. J. Ovarian Res. 2015, 8, 1–11.
- Awad, D.S.; Ali, R.M.; Mhaidat, N.M.; Shotar, A.M. Zizyphus jujuba protects against ibuprofen-induced nephrotoxicity in rats. Pharm. Biol. 2013, 52, 182–186.
- Kim, M.; Lee, E.J.; Lim, K.-M. Ibuprofen Increases the Hepatotoxicity of Ethanol through Potentiating Oxidative Stress. Biomol. Ther. 2021, 29, 205–210.
- Ma, Z.-Y.; Song, X.-Q.; Hu, J.-J.; Wang, D.-B.; Ding, X.-J.; Liu, R.-P.; Dai, M.-L.; Meng, F.-Y.; Xu, J.-Y. Ketoplatin in triple-negative breast cancer cells MDA-MB-231: High efficacy and low toxicity, and positive impact on inflammatory microenvironment. Biochem. Pharmacol. 2021, 188, 114523.
- Yasuyuki, S.; Yoshihiko, S.; Yoshio, T.; Sadao, H. Protection against cisplatin-induced nephrotoxicity in the rat by inducers and an inhibitor of glutathione S-transferase. Biochem. Pharmacol. 1994, 48, 453–459.
- Fazzio, L.; Raggio, S.; Romero, J.; Membrebe, J.; Minervino, A. Safety Study on Ketoprofen in Pigs: Evaluating the Effects of Different Dosing and Treatment Scheme on Hematological, Hepatic, and Renal Parameters. Vet. Sci. 2021, 8, 30.
- Chen, Y.; Wang, Q.; Li, Z.; Liu, Z.; Zhao, Y.; Zhang, J.; Liu, M.; Wang, Z.; Li, D.; Han, J. Naproxen platinum(iv) hybrids inhibiting cycloxygenases and matrix metalloproteinases and causing DNA damage: Synthesis and biological evaluation as antitumor agents in vitro and in vivo. Dalton Trans. 2020, 49, 5192–5204.
- Wang, Q.; Hou, X.; Gao, J.; Ren, C.; Guo, Q.; Fan, H.; Liu, J.; Zhang, W.; Liu, J. A coassembled peptide hydrogel boosts the radiosensitization of cisplatin. Chem. Commun. 2020, 56, 13017–13020.
- Li, L.; Chen, Y.; Wang, Q.; Li, Z.; Liu, Z.; Hua, X.; Han, J.; Chang, C.; Wang, Z.; Li, D. Albumin-encapsulated Nanoparticles of Naproxen Platinum(IV) Complexes with Inflammation Inhibitory Competence Displaying Effective Antitumor Activities in vitro and in vivo. Int. J. Nanomed. 2021, 16, 5513–5529.
- Jin, S.; Muhammad, N.; Sun, Y.; Tan, Y.; Yuan, H.; Song, D.; Guo, Z.; Wang, X. Multispecific Platinum(IV) Complex Deters Breast Cancer via Interposing Inflammation and Immunosuppression as an Inhibitor of COX-2 and PD-L1. Angew. Chem. Int. Ed. 2020, 59, 23313–23321.
- Poradowski, D.; Obmińska-Mrukowicz, B. Effect of selected nonsteroidal anti-inflammatory drugs on the viability of canine osteosarcoma cells of the D-17 line: In vitro studies. J. Vet. Res. 2019, 63, 399–403.
- Özdemir, Ö.; Marinelli, L.; Cacciatore, I.; Ciulla, M.; Emsen, B.; Di Stefano, A.; Mardinoglu, A.; Turkez, H. Anticancer effects of novel NSAIDs derivatives on cultured human glioblastoma cells. Zeitschrift für Naturforschung C 2021, 76, 329–335.
- Aytaç, P.; Sahin, I.D.; Atalay, R.Ç.; Tozkoparan, B. Design, Synthesis, and Biological Evaluation of Novel Triazolothiadiazoles Derived from NSAIDs as Anticancer Agents. Anti-Cancer Agents Med. Chem. 2021, 21, 1.
- Liu, F.; Wu, Q.; Han, W.; Laster, K.; Hu, Y.; Ma, F.; Chen, H.; Tian, X.; Qiao, Y.; Liu, H.; et al. Targeting integrin αvβ3 with indomethacin inhibits patient-derived xenograft tumour growth and recurrence in oesophageal squamous cell carcinoma. Clin. Transl. Med. 2021, 11, e548.
- Kozlowska, A.K.; Topchyan, P.; Kaur, K.; Tseng, H.-C.; Teruel, A.; Hiraga, T.; Jewett, A. Differentiation by NK cells is a prerequisite for effective targeting of cancer stem cells/poorly differentiated tumors by chemopreventive and chemotherapeutic drugs. J. Cancer 2017, 8, 537–554.
- Zhao, H.; Yi, B.; Liang, Z.; Phillips, C.; Lin, H.-Y.; Riker, A.I.; Xi, Y. Cyclin G2, a novel target of sulindac to inhibit cell cycle progression in colorectal cancer. Genes Dis. 2021, 8, 320–330.
- Machkalyan, G.; Hèbert, T.E.; Miller, G.J. PPIP5K1 Suppresses Etoposide-triggered Apoptosis. J. Mol. Signal. 2016, 11, 4.
- Shriwas, O.; Priyadarshini, M.; Samal, S.K.; Rath, R.; Panda, S.; Das Majumdar, S.K.; Muduly, D.K.; Botlagunta, M.; Dash, R. DDX3 modulates cisplatin resistance in OSCC through ALKBH5-mediated m6A-demethylation of FOXM1 and NANOG. Apoptosis 2020, 25, 233–246.
- Okamoto, K.; Ueda, H.; Saito, Y.; Narumi, K.; Furugen, A.; Kobayashi, M. Diclofenac potentiates the antitumor effect of cisplatin in a xenograft mouse model transplanted with cisplatin-resistant cells without enhancing cisplatin-induced nephrotoxicity. Drug Metab. Pharmacokinet. 2021, 41, 100417.
- Okamoto, K.; Saito, Y.; Narumi, K.; Furugen, A.; Iseki, K.; Kobayashi, M. Anticancer effects of non-steroidal anti-inflammatory drugs against cancer cells and cancer stem cells. Toxicol. In Vitro 2021, 74, 105155.
- Heidarian, E.; Nouri, A. Hepatoprotective effects of silymarin against diclofenac-induced liver toxicity in male rats based on biochemical parameters and histological study. Arch. Physiol. Biochem. 2021, 127, 112–118.
- Naruse, T.; Nishida, Y.; Ishiguro, N. Synergistic effects of meloxicam and conventional cytotoxic drugs in human MG-63 osteosarcoma cells. Biomed. Pharmacother. 2007, 61, 338–346.
- Honma, S.; Takahashi, N.; Shinohara, M.; Nakamura, K.; Mitazaki, S.; Abe, S.; Yoshida, M. Amelioration of cisplatin-induced mouse renal lesions by a cyclooxygenase (COX)-2 selective inhibitor. Eur. J. Pharmacol. 2013, 715, 181–188.
- Tamasi, G.; Casolaro, M.; Magnani, A.; Sega, A.; Chiasserini, L.; Messori, L.; Gabbiani, C.; Valiahdi, S.M.; Jakupec, M.A.; Keppler, B.; et al. New platinum–oxicam complexes as anti-cancer drugs. Synthesis, characterization, release studies from smart hydrogels, evaluation of reactivity with selected proteins and cytotoxic activity in vitro. J. Inorg. Biochem. 2010, 104, 799–814.
- Menale, C.; Piccolo, M.T.; Favicchia, I.; Aruta, M.G.; Baldi, A.; Nicolucci, C.; Barba, V.; Mita, D.G.; Crispi, S.; Diano, N. Efficacy of Piroxicam Plus Cisplatin-Loaded PLGA Nanoparticles in Inducing Apoptosis in Mesothelioma Cells. Pharm. Res. 2014, 32, 362–374.
- Greene, S.N.; Ramos-Vara, J.A.; Craig, B.A.; Hooser, S.B.; Anderson, C.; Fourez, L.M.; Johnson, B.M.; Stewart, J.C.; Knapp, D.W. Effects of cyclooxygenase inhibitor treatment on the renal toxicity of cisplatin in rats. Cancer Chemother. Pharmacol. 2010, 65, 549–556.
- Nilsen, O.G.; Aasarod, K.; Wideroe, T.-E.; Guentert, T.W. Single- and multiple-dose pharmacokinetics, kidney tolerability and plasma protein binding of tenoxicam in renally impaired patients and healthy volunteers. Pharmacol. Toxicol. 2001, 89, 265–272.
- Karatopuk, D.U.; Gokcimen, A. Effect of tenoxicam on rat liver tissue. Turk. J. Gastroenterol. 2010, 21, 146–152.
- Matsumoto, R.; Tsuda, M.; Yoshida, K.; Tanino, M.; Kimura, T.; Nishihara, H.; Abe, T.; Shinohara, N.; Nonomura, K.; Tanaka, S. Aldo-keto reductase 1C1 induced by interleukin-1β mediates the invasive potential and drug resistance of metastatic bladder cancer cells. Sci. Rep. 2016, 6, 34625.
- Shiiba, M.; Yamagami, H.; Yamamoto, A.; Minakawa, Y.; Okamoto, A.; Kasamatsu, A.; Sakamoto, Y.; Uzawa, K.; Takiguchi, Y.; Tanzawa, H. Mefenamic acid enhances anticancer drug sensitivity via inhibition of aldo-keto reductase 1C enzyme activity. Oncol. Rep. 2017, 37, 2025–2032.
- Soriano-Hernandez, A.D.; Madrigal-Pérez, D.; Galvan-Salazar, H.R.; Martinez-Fierro, M.L.; Valdez, L.; Gomez, F.E.; Vazquez-Vuelvas, O.F.; Olmedo-Buenrostro, B.A.; Guzman-Esquivel, J.; Rodriguez-Sanchez, I.P.; et al. Anti-inflammatory drugs and uterine cervical cancer cells: Antineoplastic effect of meclofenamic acid. Oncol. Lett. 2015, 10, 2574–2578.
- Caglar, S.; Altay, A.; Kuzucu, M.; Caglar, B. In Vitro Anticancer Activity of Novel Co(II) and Ni(II) Complexes of Non-steroidal Anti-inflammatory Drug Niflumic Acid Against Human Breast Adenocarcinoma MCF-7 Cells. Cell Biophys. 2021, 79, 729–746.
- Zhou, P.; Wu, M.; Ye, C.; Xu, Q.; Wang, L. Meclofenamic acid promotes cisplatin-induced acute kidney injury by inhibiting fat mass and obesity-associated protein-mediated m6A abrogation in RNA. J. Biol. Chem. 2019, 294, 16908–16917.
- Li, H.; Song, Y.; He, Z.; Chen, X.; Wu, X.; Li, X.; Bai, X.; Liu, W.; Li, B.; Wang, S.; et al. Meclofenamic Acid Reduces Reactive Oxygen Species Accumulation and Apoptosis, Inhibits Excessive Autophagy, and Protects Hair Cell-Like HEI-OC1 Cells from Cisplatin-Induced Damage. Front. Cell. Neurosci. 2018, 12, 139.
- Grande, F.; Giordano, F.; Occhiuzzi, M.; Rocca, C.; Ioele, G.; De Luca, M.; Ragno, G.; Panno, M.; Rizzuti, B.; Garofalo, A. Toward Multitasking Pharmacological COX-Targeting Agents: Non-Steroidal Anti-Inflammatory Prodrugs with Antiproliferative Effects. Molecules 2021, 26, 3940.
- Arai, Y.; Tanaka, K.-I.; Ushijima, H.; Tomisato, W.; Tsutsumi, S.; Aburaya, M.; Hoshino, T.; Yokomizo, K.; Suzuki, K.; Katsu, T.; et al. Low Direct Cytotoxicity of Nabumetone on Gastric Mucosal Cells. Dig. Dis. Sci. 2005, 50, 1641–1646.
- Frejborg, E.; Salo, T.; Salem, A. Role of Cyclooxygenase-2 in Head and Neck Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 9246.
- Li, W.; Zhang, Z.; Wang, B.; Liang, N.; Zhou, Q.; Long, S. MicroRNA and cyclooxygenase-2 in breast cancer. Clin. Chim. Acta 2021, 522, 36–44.
- Zhu, F.S.; Chen, X.M.; Huang, Z.G.; Wang, Z.R.; Zhang, D.W.; Zhang, X. Rofecoxib augments anticancer effects by reversing intrinsic multidrug resistance gene expression in BGC-823 gastric cancer cells. J. Dig. Dis. 2010, 11, 34–42.
- Yu, L.; Chen, M.; Li, Z.; Wen, J.; Fu, J.; Guo, D.; Jiang, Y.; Wu, S.; Cho, C.-H.; Liu, S. Celecoxib Antagonizes the Cytotoxicity of Cisplatin in Human Esophageal Squamous Cell Carcinoma Cells by Reducing Intracellular Cisplatin Accumulation. Mol. Pharmacol. 2010, 79, 608–617.
- Arora, M.; Choudhary, S.; Singh, P.K.; Sapra, B.; Silakari, O. Structural investigation on the selective COX-2 inhibitors mediated cardiotoxicity: A review. Life Sci. 2020, 251, 117631.
- Sun, S.X.; Lee, K.Y.; Bertram, C.T.; Goldstein, J.L. Withdrawal of COX-2 selective inhibitors rofecoxib and valdecoxib: Impact on NSAID and gastroprotective drug prescribing and utilization. Curr. Med Res. Opin. 2007, 23, 1859–1866.
- Brenner, G.B.; Makkos, A.; Nagy, C.T.; Onódi, Z.; Sayour, N.V.; Gergely, T.G.; Kiss, B.; Görbe, A.; Sághy, É.; Zádori, Z.S.; et al. Hidden Cardiotoxicity of Rofecoxib Can be Revealed in Experimental Models of Ischemia/Reperfusion. Cells 2020, 9, 551.
- Atashbar, S.; Jamali, Z.; Khezri, S.; Salimi, A. Celecoxib decreases mitochondrial complex IV activity and induces oxidative stress in isolated rat heart mitochondria: An analysis for its cardiotoxic adverse effect. J. Biochem. Mol. Toxicol. 2021, 2021, e22934.
- Wu, F.; Wang, W.; Duan, Y.; Guo, J.; Li, G.; Ma, T. Effect of Parecoxib Sodium on Myocardial Ischemia-Reperfusion Injury Rats. Med. Sci. Monit. 2020, 27, e928205-1.
- Okamoto, K.; Saito, Y.; Narumi, K.; Furugen, A.; Iseki, K.; Kobayashi, M. Comparison of the nephroprotective effects of non-steroidal anti-inflammatory drugs on cisplatin-induced nephrotoxicity in vitro and in vivo. Eur. J. Pharmacol. 2020, 884, 173339.
- El-Kader, M.A.; Taha, R.I. Comparative nephroprotective effects of curcumin and etoricoxib against cisplatin-induced acute kidney injury in rats. Acta Histochem. 2020, 122, 151534.
