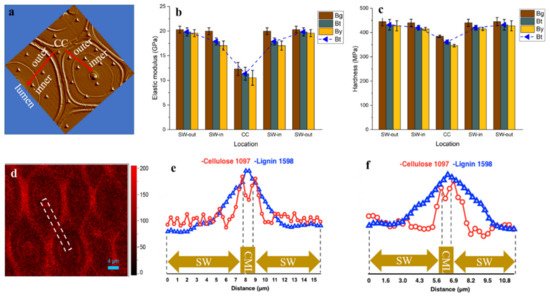
The mechanical performance of bamboo is highly dependent on its structural arrangement and the properties of biomacromolecules within the cell wall. Along the radius of bamboo culms, the concentration of xylan within the fiber sheath increased, while that of cellulose and lignin decreased gradually. At cellular level, although the consecutive broad layer (Bl) of fiber revealed a relatively uniform cellulose orientation and concentration, the outer Bl with higher lignification level has higher elastic modulus (19.59–20.31 GPa) than that of the inner Bl close to the lumen area (17.07–19.99 GPa). Comparatively, the cell corner displayed the highest lignification level, while its hardness and modulus were lower than that of fiber Bl, indicating the cellulose skeleton is the prerequisite of cell wall mechanics. The obtained cytological information is helpful to understand the origin of the anisotropic mechanical properties of bamboo.
- multilayered bamboo fiber
- topochemistry
- microscopic imaging
- gradient micromechanics
1. Introduction
In terms of tissue types, the bamboo culm consists of fibers, parenchyma and conducting elements (including the xylem vessels, sieve tubes, and companion cells). As the source of the superior mechanical properties of bamboo, the fibers’ density and quality vary significantly along and across the bamboo culm. For example, along the diametric direction, the longitudinal tensile modulus of elasticity for the outermost layer was 3–4 times as high as that of the innermost layer, while the longitudinal tensile strength ranged from 115.94 to 328.15 MPa from the outermost layer to the innermost layer [6][1].
Actually, much of the mechanical behavior of bamboo is governed by the properties of the cell wall, which, in turn, can be described in terms of the submicroscopic structure of the wall and the localization of cell wall components of cellulose, hemicelluloses, and lignin. Cellulose is the main structural fiber in the plant kingdom and has remarkable mechanical properties for a polymer: its Young’s modulus is roughly 130 GPa, and its tensile strength is close to and even more than 1 GPa [10][2]. The properties of hemicelluloses and lignin are similar to common engineering polymers. Lignin, for instance, has a modulus of roughly 3 GPa and a strength of about 50 MPa [11][3]. An important role of lignin in the wood cell wall is to function as a cross-linking matrix between moisture sensitive cellulose and hemicelluloses, thereby, lignin contributes to the mechanical rigidity [12][4]. The modulus of xylan varies from a value of 8 GPa at low moisture contents (0–10%) to 10 MPa at moisture contents near saturation (70%). Meanwhile, the incorporation of xylan in the cell wall, especially in the secondary wall, has a strength-enhancing effect on the joint strength individual fiber crossings [13][5]. The arrangement of the basic building blocks in bamboo cell walls and the variations in cellular structure give rise to a remarkably wide range of mechanical properties.
Unlike the typical three-layered structure of wood secondary wall, bamboo exhibits a polylamellate secondary wall with alternating broad and narrow lamellae that arise from the alternation in the orientation of cellulose microfibrils in a matrix of intertwined hemicelluloses and lignin. To date, macro- and micro-structural investigations have been reported comprehensively.
2. FT-IR Chemical Image of Bamboo Culm
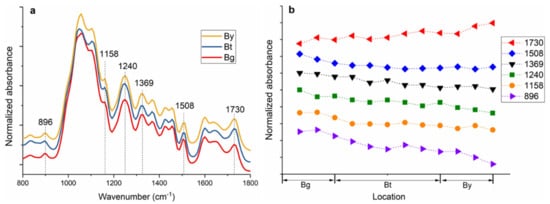
The FT-IR spectra of the bamboo fiber sheaths extracted from Bg, Bt and By are shown in Figure 1a. FT-IR spectra indicated peak changes in fingerprint regions at various tissues and cell locations. The main differences in the absorption spectra are visible at wavenumbers 1730, 1508, 1369, 1240, 1158, and 896 cm−1. The IR band at 1508 cm−1 corresponds to a C=C stretching the vibration of the aromatic rings of lignin. The carbohydrates peaks at 1730, 1369, and 1158 cm−1 are assigned, respectively, for unconjugated C=O in xylan, C-H deformation in cellulose and hemicelluloses, and C–O–C vibration in cellulose and hemicelluloses [17,18][6][7]. Moreover, the band at 1240 and 896 cm−1 is a diagnostic peak for cellulose by the C–O–C vibration and C–H deformation in cellulose, respectively [19,20][8][9]. The relative absorbance of characteristic FT-IR absorbance bands is plotted as a function of the fiber sheaths (Figure 1b).
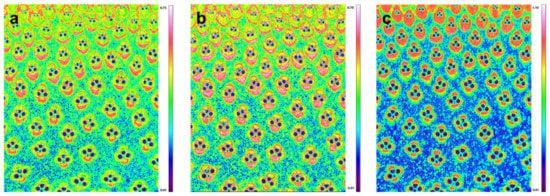
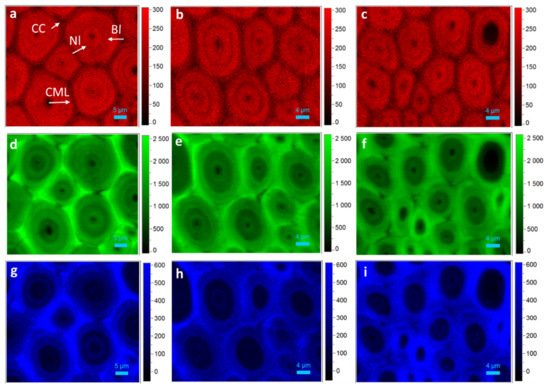
3. Confocal Raman Chemical Image of Bamboo Fiber
4. The Variation in the Mechanical Properties of Fiber Cell Walls
5. Conclusions
References
- Yu, H.Q.; Fei, B.H.; Ren, H.Q.; Jiang, Z.H.; Liu, X.E. Variation in tensile properties and relationship between tensile properties and air-dried density for Moso bamboo. Front. For. China 2008, 3, 127–130.
- Mittal, N.; Ansari, F.; Gowda, V.K.; Brouzet, C.; Chen, P.; Larsson, P.T.; Toth, S.V.; Lundell, F.; Wågberg, L.; Kotov, N.A.; et al. Multiscale control of nanocellulose assembly: Transferring remarkable nanoscale fibril mechanics to macroscale fibers. ACS Nano 2018, 12, 6378–6388.
- Gibbson, L.J. The hierarchical structure and mechanics of plant materials. J. R. Soc. Interface 2012, 9, 2749–2766.
- Salmén, L.; Burgert, I. Cell wall features with regard to mechanical performance. A review COST Action E35 2004-2008: Wood machining micromechanics and fracture. Holzforschung 2009, 63, 121–129.
- Miletzky, A.; Fischer, W.J.; Czibula, C.; Teichert, C.; Bauer, W.; Schennach, R. How xylan effects the breaking load of individual fiber-fiber joints and the single fiber tensile strength. Cellulose 2015, 22, 849–859.
- Kačuráková, M.; Wellner, N.; Ebringerová, A.; Hromádková, Z.; Wilson, R.H.; Belton, P.S. Characterisation of xylan type polysaccharides and associated cell wall components by FT-IR and FT-Raman spectroscopies. Food Hydrocolloid 1999, 13, 35–41.
- Chang, S.S.; Salmén, L.; Olsson, A.M.; Clair, B. Deposition and organisation of cell wall polymers during maturation of poplar tension wood by FT-IR microspectroscopy. Planta 2014, 239, 243–254.
- Michell, A.J. 2nd-derivative FT-IR spectra of native celluloses. Carbohyd. Res. 1990, 197, 53–60.
- Peng, H.; Salmén, L.; Stevanic, J.S.; Lu, J.X. Structural organization of the cell wall polymers in compression wood as revealed by FTIR microspectroscopy. Planta 2019, 250, 163–171.
- Domínguezrobles, J.; Sánchez, R.; Espinosa, E.; Savy, D.; Mazzei, P.; Piccolo, A.; Rodríguez, A. Isolation and characterization of gramineae and fabaceae soda lignins. Int. J. Mol. Sci. 2017, 18, 327.
- Yuan, Z.; Wen, Y. Evaluation of an integrated process to fully utilize bamboo biomass during the production of bioethanol. Bioresour. Technol. 2017, 236, 202–211.
- Li, Z.Q.; Jiang, Z.H.; Fei, B.H.; Cai, Z.Y.; Pan, X.J. Comparison of bamboo green, timber and yellow in sulfite, sulfuric acid and sodium hydroxide pretreatments for enzymatic saccharification. Bioresour. Technol. 2014, 151, 91–99.
- Zou, L.; Jin, H.; Lu, W.Y.; Li, X. Nanoscale structural and mechanical characterization of the cell wall of bamboo fibers. Mat. Sci. Eng. C-Mater. 2009, 29, 1375–1379.
- Jäger, A.; Bader, T.; Hofstetter, K.; Eberhardsteiner, J. The relation between indentation modulus, microfibril angle, and elastic properties of wood cell walls. Compos. Part A-Appl. Sci. 2011, 42, 677–685.
- Yu, Y.; Fei, B.; Zhang, B.; Yu, X. Cell-wall mechanical properties of bamboo investigated by in-situ imaging nanoindentation. Wood Fiber Sci. 2007, 39, 527–535.
- Parameswaran, N.; Liese, W. On the fine structure of bamboo fibres. Wood Sci. Technol. 1976, 10, 231–264.
- Gindl, W.; Gupta, H.S.; Grünwald, C. Lignification of spruce tracheid secondary cell walls related to longitudinal hardness and modulus of elasticity using nano-indentation. Can. J. Bot. 2002, 80, 1029–1033.
