Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Xuerong Li and Version 2 by Vivi Li.
Pomacea canaliculata is one of the 100 worst invasive alien species in the world, which has significant effects and harm to native species, ecological environment, human health, and social economy. Climate change is one of the major causes of species range shifts. With recent climate change, the distribution of P. canaliculata has shifted northward. Understanding the potential distribution under current and future climate conditions will aid in the management of the risk of its invasion and spread.
- MaxEnt
- Pomacea canaliculata
- biological invasion
- dispersal risk
1. Introduction
Heightened connectivity between countries brought about by globalization’s facilitation has contributed to tremendous economic and social development through global trade, international travel/tourism, etc. and also resulted in the introduction of numerous invasive alien species, posing significant threats to native species, the ecological environment, human health, and the social economy [1].
Pomacea canaliculata (Gastropoda: Ampullariidae), commonly called the apple snail, a freshwater snail native to tropical and temperate South America, was listed by the International Union of Conservation of Nature (IUCN) in the “100 of the world’s worst invasive alien species” [2], as well as among the first batch of invasive alien species in China. The invasion of P. canaliculata severely harmed the biodiversity in China, altered the spatial distribution of native species, caused direct harm to the production of agriculture, forestry, animal husbandry, and fishery, and resulted in massive economic losses [3]. The species can also lead to disease spread and pose serious threats as pathogen vectors to China’s public health security [4].
Some species of Pomacea have characteristics that have been linked to invasiveness. After invading the United States, Japan, the Philippines, and other countries, it caused serious damage to the local nature and agriculture, but the control effect of various countries was not ideal, and the population and spread area continued to increase [5][6][5,6]. P. canaliculata, which is widely distributed in China’s south of the Yangtze River, is the most common Pomacea spp. Found in the area [7][8][7,8]. Due to its omnivorous habits and large food intake, it primarily harms aquatic crops such as rice [9], which is the main alimentary crop in China. At the same time, the feeding of P. canaliculata is selective, endangering the species diversity of the aquatic plant community [10]. Its excretory–secretory products can pollute the water quality environment and contribute to water body eutrophication [11]. With characteristics such as strong adaptability, rapid reproduction, and also resistance to high temperature, hypoxia, cold, hunger, acid and alkali, water pollution, etc. [12][13][14][15][12,13,14,15], P. canaliculata more easily becomes the local dominant population, causing varying degrees of damage to the fish and shellfish resources in the water and endangering the local biodiversity. Meanwhile, as an intermediate host, it carries several main parasites that are harmful to human health and include Echinostomarevolutum, Angiostrongylus cantonensis, and Gonathostomaspinigerum, which cause a variety of serious diseases such as echinostomiasis, eosinophilic meningitis, gnathostomiasis, etc. [16][17][16,17]. Although A. cantonensis primarily uses Achatina fulica and P. canaliculata as intermediate hosts [18], eosinophilic meningitis caused by P. canaliculata is more common due to its large market sales volume, widespread distribution, and strong adaptability. In an outbreak of A. cantonensis in Beijing during 2006, as many as 160 patients became ill after eating undercooked P. canaliculata or related eatables, showing varying degrees of fever, headache, neck stiffness, and skin paresthesia [19].
Quantitative risk assessment of alien invasive species is the general trend of development. Currently, risk assessment is based on the analysis of the adaptability of alien invasive species in the target area. Species distribution modeling (SDM) has been widely used in assessing the risk of invasive alien species [20], as well as in simulating pest and disease spread [21]. It estimates its potential distribution in the target area, using species distribution data and environmental variables, and evaluates the importance of environmental variables using jackknife. Due to the accuracy of prediction, particularly in the case of few or incomplete distribution data, the maximum entropy (MaxEnt) model is one of the most commonly used models [22]. Furthermore, the kuenm package makes use of the flexibility of R and MaxEnt to allow for detailed model calibration and selection, final model development and evaluation, and extrapolation risk analysis [23]. The MaxEnt model optimized via kuenm could better predict the distribution of P. canaliculata, which will limit the future development of the species to some extent, with corresponding policies for prevention and control.
2. Environmental Variables and Model Optimization
Among 248 candidates, only one statistically significant model met the omission rate and AICc criteria. In this candidate model (RM = 0.5 FC = LQP), the mean AUC ratio was 1.782, the partial ROC was 0, the omission rate was 0.05, and the AICc was 10991.12, which represents the lowest delta AICc after adjusting (Supplementary Table S2, could be found in https://www.mdpi.com/2079-7737/11/1/110#supplementary).
Pearson’s correlation coefficients among 11 main contribution variables are shown in Figure 1 and Supplementary Table S3. The relative contributions of the environmental variables to the MaxEnt model were estimated, and seven environmental variables, including Bio8, bio12, bio18, bio19, elev, prec3, and tmax11, were ultimately chosen for generating the model (Table 1).
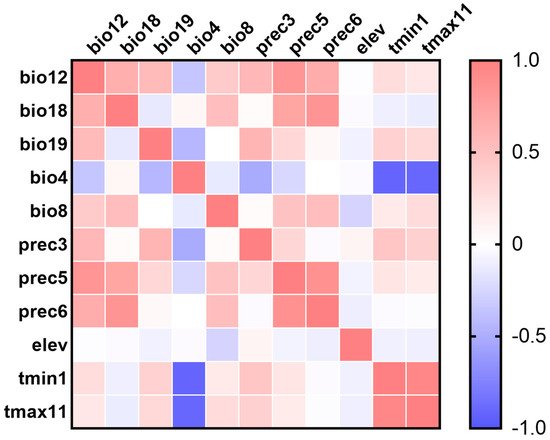

Figure 1. Pearson’s correlation matrix heatmap of environmental variables with contribution percentage greater than 1%. The variables include bio12 (annual precipitation), bio18 (precipitation of warmest quarter), bio19 (precipitation of coldest quarter), bio4 (temperature Seasonality (standard deviation × 100)), bio8 (mean temperature of wettest quarter), prec3 (precipitation of March), prec5 (precipitation of May), prec6 (precipitation of June), elev (elevation), tmin1 (minimum temperature of January), tmax11 (maximum temperature of November).
Table 1. Percent contribution and permutation importance of the environmental variables in the MaxEnt model.
| Code | Environmental Variables | Percent Contribution | Permutation Importance |
|---|---|---|---|
| Bio18 | Precipitation of warmest quarter | 42.4 | 9.6 |
| Tmax11 | Maximum temperature of November | 29.6 | 53.4 |
| Elev | Elevation | 17.6 | 19.2 |
| Bio8 | Mean temperature of wettest quarter | 5.5 | 15.1 |
| Bio12 | Annual precipitation | 1.9 | 0.5 |
| Prec3 | Precipitation of March | 1.5 | 0.8 |
| Bio19 | Precipitation of coldest quarter | 1.4 | 1.3 |
3. Current Prediction of P. canaliculata
The potential distribution was predicted based on the above models and current environmental variables, which occurred in the south of the Yangtze River, as well as in the most southeastern part of China (Figure 2). The highly suitable, moderately suitable, and low-suitable habitats accounted for 5.62%, 8.486%, and 7.50%, respectively. The average AUC value of repeated operation was 0.962, and the standard deviation (SD) was 0.002, indicating that the model is highly reliable for the potential habitat of P. canaliculata and can effectively reflect its distribution in China (Figure 3).
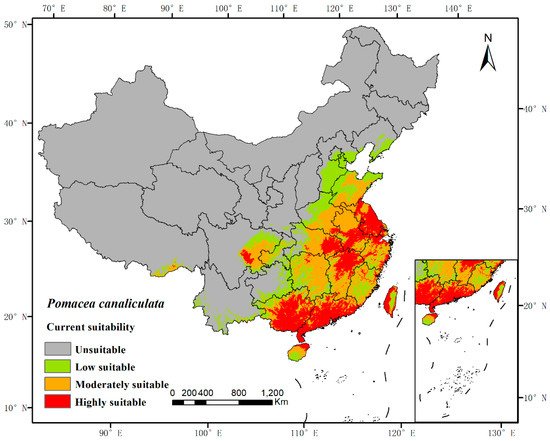
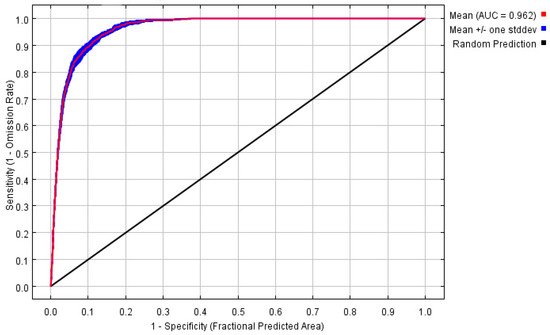

Figure 2. MaxEnt model predicted the current potential habitat suitability of Pomacea canaliculata in China. The base map was obtained from the Ministry of Natural Resources of China (http://bzdt.ch.mnr.gov.cn/index.html, accessed on 5 November 2021).

Figure 3. The receiver operating characteristic (ROC) curve and average area under curve (AUC) values for the optimized model over 10 replicate runs were shown in red, while blue margins show ± standard deviation (SD) calculated for 10 replicates.
According to the results of the jackknife test of variable importance (Figure 4), bio18 had the greatest influence on the distribution of P. canaliculata, followed by tamx11. The cumulative contribution of the two variables was more than 70%, which were major factors that contributed to the MaxEnt model.
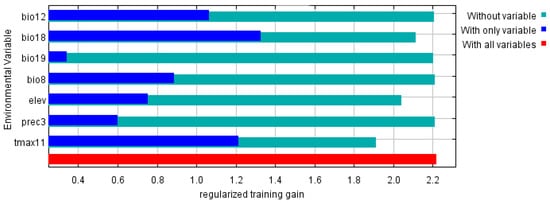

Figure 4. Jackknife test of variable importance in the P. canaliculata suitability distribution.
The response curves showed how the predicted probability of presence changes as each environmental variable was varied (Figure 5). A probability value greater than MTSS indicated that the environment was suitable for the growth of P. canaliculata. As a result, the suitable range for the precipitation of the warmest quarter was 230.89~2044.43 mm, the suitable range for a maximum temperature of November was 7.86~33.17 °C, the suitable range for elevation was less than 607.44 m, the suitable range for a mean temperature of the wettest quarter was more than 13.96 °C, the suitable range for annual precipitation was 574.41~3803.38 mm, the suitable range for precipitation of March was 5.94~359.50 mm, and the suitable range for precipitation of the coldest quarter were less than 1076.88 mm.
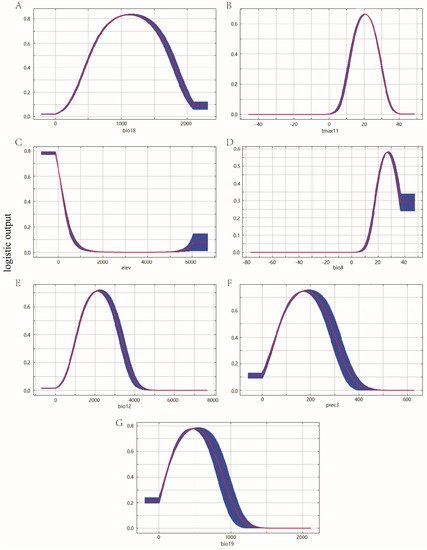

Figure 5. Response curves of environmental variables in the potential distribution model of P. canaliculata. The red curves represent average value over 10 replicate runs, while blue margins represented ± SD calculated for 10 replicates: (A) Bio18, (B) Tmax11, (C) Elev, (D) Bio8, (E) Bio12, (F) Prec3, and (G) Bio19.
4. Future Prediction of P. canaliculata
The change of P. canaliculata potential distribution from 2021 to 2100 in four SSPs of CMIP6 is shown in Figure 6 (Supplementary Table S4). This model predicted that global warming would promote the expansion of the potentially suitable habitats of P. canaliculata, with the total suitable habitats increasing (Supplementary Table S5 and Figure 7). Furthermore, the centroid would move from south to north, particularly in SSP585 (Figure 8).
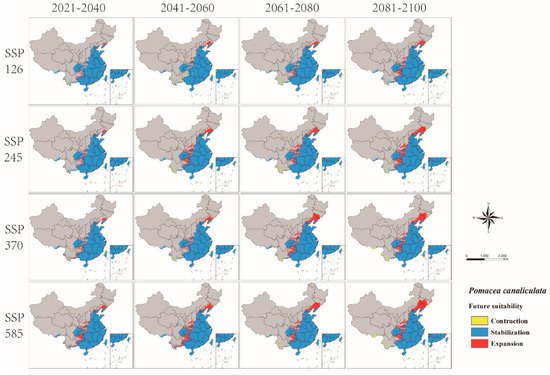
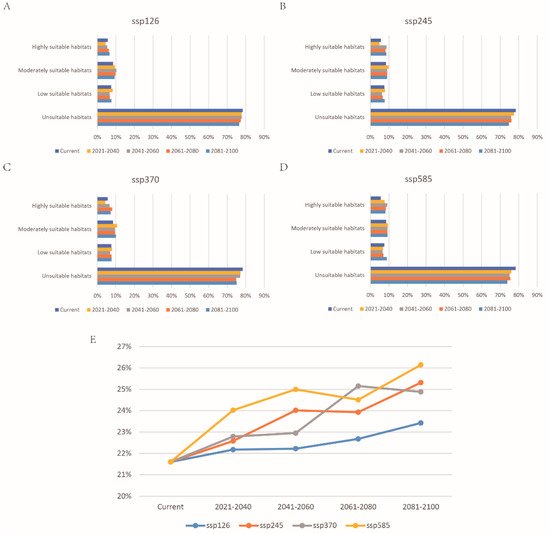
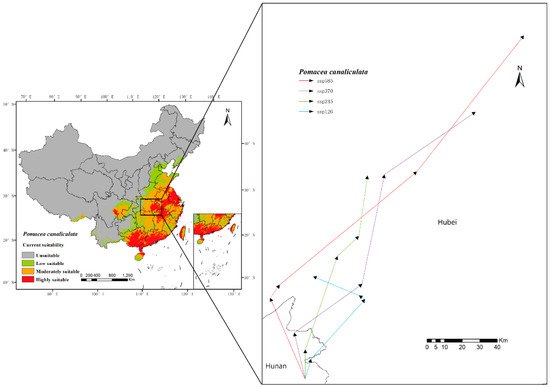

Figure 6. Changes in the potential distribution of P. canaliculata in China from 2021 to 2100 under four shared socioeconomic pathways (SSPs).

Figure 7. The proportion of suitable habitats for P. canaliculata under current and future climate change scenarios in China: (A) SSP126, (B) SSP245, (C) SSP370, and (D) SSP585; (E) change in the proportion of total suitable habitats (including low suitable, moderately suitable, and highly suitable habitats).

Figure 8. Change in distribution centroid under four SSPs in China from 2021 to 2100, with the distribution centroid shifting from the north of Hunan Province to Hubei Province.
