Immunotherapy has made great progress in recent years, yet the efficacy of solid tumors remains far less than expected. One of the main hurdles is to overcome the immune-suppressive tumor microenvironment (TME). Among all cells in TME, tumor-associated macrophages (TAMs) play pivotal roles because of their abundance, multifaceted interactions to adaptive and host immune systems, as well as their context-dependent plasticity. Underlying the highly plastic characteristic, lots of research interests are focused on repolarizing TAMs from M2-like pro-tumor phenotype towards M1-like antitumoral ones. Nanotechnology offers great opportunities for targeting and modulating TAM polarization to mount the therapeutic efficacy in cancer immunotherapy.
- macrophage polarization
- tumor-associated macrophages
- nanoparticles
- drug delivery
- tumor microenvironment
- cancer immunotherapy
1. Polymeric Nanoparticles and Macrophage Repolarization
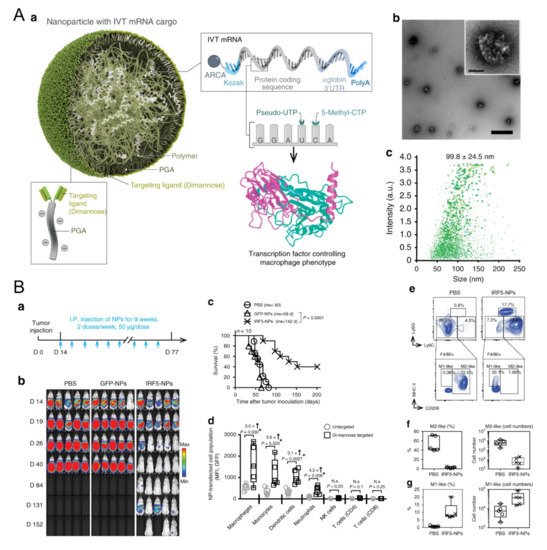
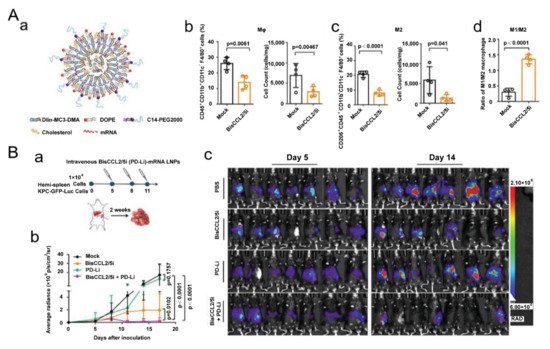
2. Lipid-Based Nanomaterials and Macrophage Repolarization
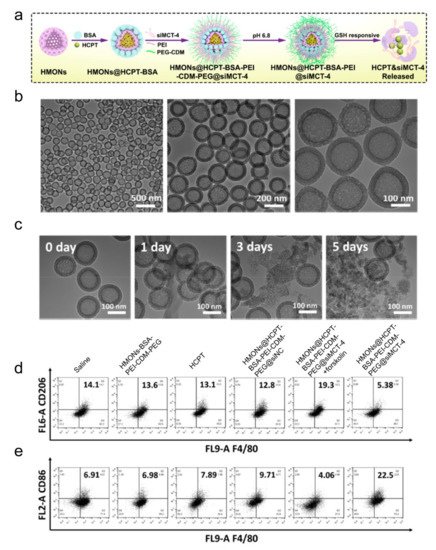
3. Inorganic Nanoparticles and Macrophage Repolarization
References
- Fuchs, A.K.; Syrovets, T.; Haas, K.A.; Loos, C.; Musyanovych, A.; Mailander, V.; Landfester, K.; Simmet, T. Carboxyl- and amino-functionalized polystyrene nanoparticles differentially affect the polarization profile of M1 and M2 macrophage subsets. Biomaterials 2016, 85, 78–87.
- Huang, Y.J.; Hung, K.C.; Hung, H.S.; Hsu, S.H. Modulation of Macrophage Phenotype by Biodegradable Polyurethane Nanoparticles: Possible Relation between Macrophage Polarization and Immune Response of Nanoparticles. Appl. Mater Interfaces 2018, 10, 19436–19448.
- Zhou, J.; Kroll, A.V.; Holay, M.; Fang, R.H.; Zhang, L. Biomimetic Nanotechnology toward Personalized Vaccines. Adv Mater 2019, e1901255.
- Fang, R.H.; Jiang, Y.; Fang, J.C.; Zhang, L. Cell memb.brane-derived nanomaterials for biomedical applications. Biomaterials 2017, 128, 69–83.
- Deng, G.; Sun, Z.; Li, S.; Peng, X.; Li, W.; Zhou, L.; Ma, Y.; Gong, P.; Cai, L. Cell-Membrane Immunotherapy Based on Natural Killer Cell Membrane Coated Nanoparticles for the Effective Inhibition of Primary and Abscopal Tumor Growth. ACS Nano 2018, 12, 12096–12108.
- Liu, L.; Wang, Y.; Guo, X.; Zhao, J.; Zhou, S. A Biomimetic Polymer Magnetic Nanocarrier Polarizing Tumor-Associated Macrophages for Potentiating Immunotherapy. Small 2020, e2003543.
- Da Silva, C.G.; Camps, M.G.M.; Li, T.; Chan, A.B.; Ossendorp, F.; Cruz, L.J. Co-delivery of immunomodulators in biodegradable nanoparticles improves therapeutic efficacy of cancer vaccines. Biomaterials 2019, 220, 119417.
- Rodell, C.B.; Arlauckas, S.P.; Cuccarese, M.F.; Garris, C.S.; Li, R.; Ahmed, M.S.; Kohler, R.H.; Pittet, M.J.; Weissleder, R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588.
- Figueiredo, P.; Lepland, A.; Scodeller, P.; Fontana, F.; Torrieri, G.; Tiboni, M.; Shahbazi, M.A.; Casettari, L.; Kostiainen, M.A.; Hirvonen, J.; et al. Peptide-guided resiquimod-loaded lignin nanoparticles convert tumor-associated macrophages from M2 to M1 phenotype for enhanced chemotherapy. Acta Biomater. 2020.
- Wei, X.; Liu, L.; Li, X.; Wang, Y.; Guo, X.; Zhao, J.; Zhou, S. Selectively targeting tumor-associated macrophages and tumor cells with polymeric micelles for enhanced cancer chemo-immunotherapy. J. Control. Release 2019, 313, 42–53.
- Nam, J.; Son, S.; Park, K.S.; Moon, J.J. Modularly Programmable Nanoparticle Vaccine Based on Polyethyleneimine for Personalized Cancer Immunotherapy. Adv. Sci. 2021, 8, 2002577.
- Qiu, N.; Wang, G.; Wang, J.; Zhou, Q.; Guo, M.; Wang, Y.; Hu, X.; Zhou, H.; Bai, R.; You, M.; et al. Tumor-Associated Macrophage and Tumor-Cell Dually Transfecting Polyplexes for Efficient Interleukin-12 Cancer Gene Therapy. Adv. Mater. 2021, 33, e2006189.
- Zhang, F.; Parayath, N.N.; Ene, C.I.; Stephan, S.B.; Koehne, A.L.; Coon, M.E.; Holland, E.C.; Stephan, M.T. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat. Commun. 2019, 10, 3974.
- Song, Y.; Tang, C.; Yin, C. Combination antitumor immunotherapy with VEGF and PIGF siRNA via systemic delivery of multi-functionalized nanoparticles to tumor-associated macrophages and breast cancer cells. Biomaterials 2018, 185, 117–132.
- Parayath, N.N.; Parikh, A.; Amiji, M.M. Repolarization of Tumor-Associated Macrophages in a Genetically Engineered Nonsmall Cell Lung Cancer Model by Intraperitoneal Administration of Hyaluronic Acid-Based Nanoparticles Encapsulating MicroRNA-125b. Nano Lett. 2018, 18, 3571–3579.
- Zimel, M.N.; Horowitz, C.B.; Rajasekhar, V.K.; Christ, A.B.; Wei, X.; Wu, J.; Wojnarowicz, P.M.; Wang, D.; Goldring, S.R.; Purdue, P.E.; et al. HPMA-Copolymer Nanocarrier Targets Tumor-Associated Macrophages in Primary and Metastatic Breast Cancer. Mol. Cancer 2017, 16, 2701–2710.
- Guo, Q.; He, X.; Li, C.; He, Y.; Peng, Y.; Zhang, Y.; Lu, Y.; Chen, X.; Zhang, Y.; Chen, Q.; et al. Dandelion-Like Tailorable Nanoparticles for Tumor Microenvironment Modulation. Adv. Sci. 2019, 6, 1901430.
- Wang, J.; Shen, S.; Li, J.; Cao, Z.; Yang, X. Precise Depletion of Tumor Seed and Growing Soil with Shrinkable Nanocarrier for Potentiated Cancer Chemoimmunotherapy. ACS Nano 2021, 15, 4636–4646.
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218.
- Yuba, E. Liposome-based immunity-inducing systems for cancer immunotherapy. Mol. Immunol. 2018, 98, 8–12.
- Boulikas, T. Clinical overview on Lipoplatin: A successful liposomal formulation of cisplatin. Expert Opin. Investig. Drugs 2009, 18, 1197–1218.
- Damiati, S.; Kompella, U.B.; Damiati, S.A.; Kodzius, R. Microfluidic Devices for Drug Delivery Systems and Drug Screening. Genes 2018, 9, 103.
- Sousa, S.; Auriola, S.; Monkkonen, J.; Maatta, J. Liposome encapsulated zoledronate favours M1-like behaviour in murine macrophages cultured with soluble factors from breast cancer cells. BMC Cancer 2015, 15, 4.
- Rajan, R.; Sabnani, M.K.; Mavinkurve, V.; Shmeeda, H.; Mansouri, H.; Bonkoungou, S.; Le, A.D.; Wood, L.M.; Gabizon, A.A.; La-Beck, N.M. Liposome-induced immunosuppression and tumor growth is mediated by macrophages and mitigated by liposome-encapsulated alendronate. J. Control. Release 2018, 271, 139–148.
- Shobaki, N.; Sato, Y.; Suzuki, Y.; Okabe, N.; Harashima, H. Manipulating the function of tumor-associated macrophages by siRNA-loaded lipid nanoparticles for cancer immunotherapy. J. Control. Release 2020, 325, 235–248.
- Li, H.; Somiya, M.; Kuroda, S.i. Enhancing antibody-dependent cellular phagocytosis by Re-education of tumor-associated macrophages with resiquimod-encapsulated liposomes. Biomaterials 2021, 268, 120601.
- Wang, Y.; Tiruthani, K.; Li, S.; Hu, M.; Zhong, G.; Tang, Y.; Roy, S.; Zhang, L.; Tan, J.; Liao, C.; et al. mRNA Delivery of a Bispecific Single-Domain Antibody to Polarize Tumor-Associated Macrophages and Synergize Immunotherapy against Liver Malignancies. Adv. Mater. 2021.
- Ramesh, A.; Kumar, S.; Nandi, D.; Kulkarni, A. CSF1R- and SHP2-Inhibitor-Loaded Nanoparticles Enhance Cytotoxic Activity and Phagocytosis in Tumor-Associated Macrophages. Adv. Mater. 2019, e1904364.
- Ramesh, A.; Brouillard, A.; Kumar, S.; Nandi, D.; Kulkarni, A. Dual inhibition of CSF1R and MAPK pathways using supramolecular nanoparticles enhances macrophage immunotherapy. Biomaterials 2019, 227, 119559.
- Tu, B.; He, Y.; Chen, B.; Wang, Y.; Gao, Y.; Shi, M.; Liu, T.; Asrorov, A.M.; Huang, Y. Deformable liposomal codelivery of vorinostat and simvastatin promotes antitumor responses through remodeling tumor microenvironment. Biomater. Sci. 2020, 8, 7166–7176.
- Tang, X.; Sui, D.; Liu, M.; Zhang, H.; Liu, M.; Wang, S.; Zhao, D.; Sun, W.; Liu, M.; Luo, X.; et al. Targeted delivery of zoledronic acid through the sialic acid—Siglec axis for killing and reversal of M2 phenotypic tumor-associated macrophages—A promising cancer immunotherapy. Int. J. Pharm. 2020, 590, 119929.
- Kim, S.Y.; Kim, S.; Kim, J.E.; Lee, S.N.; Shin, I.W.; Shin, H.S.; Jin, S.M.; Noh, Y.W.; Kang, Y.J.; Kim, Y.S.; et al. Lyophilizable and Multifaceted Toll-like Receptor 7/8 Agonist-Loaded Nanoemulsion for the Reprogramming of Tumor Microenvironments and Enhanced Cancer Immunotherapy. ACS Nano 2019, 13, 12671–12686.
- Ye, H.; He, X.; Feng, X. Developing neobavaisoflavone nanoemulsion suppresses lung cancer progression by regulating tumor microenvironment. Biomed. Pharm. 2020, 129, 110369.
- Das, A.; Ali, N. Nanovaccine: An emerging strategy. Expert Rev. Vaccines 2021, 20, 1273–1290.
- Jiao, M.; Zhang, P.; Meng, J.; Li, Y.; Liu, C.; Luo, X.; Gao, M. Recent advancements in biocompatible inorganic nanoparticles towards biomedical applications. Biomater. Sci. 2018, 6, 726–745.
- Wang, X.; Zhong, X.; Li, J.; Liu, Z.; Cheng, L. Inorganic nanomaterials with rapid clearance for biomedical applications. Chem. Soc. Rev. 2021, 50, 8669–8742.
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387.
- Shah, A.; Dobrovolskaia, M.A. Immunological effects of iron oxide nanoparticles and iron-based complex drug formulations: Therapeutic benefits, toxicity, mechanistic insights, and translational considerations. Nanomedicine 2018, 14, 977–990.
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994.
- Jin, R.; Liu, L.; Zhu, W.; Li, D.; Yang, L.; Duan, J.; Cai, Z.; Nie, Y.; Zhang, Y.; Gong, Q.; et al. Iron oxide nanoparticles promote macrophage autophagy and inflammatory response through activation of toll-like Receptor-4 signaling. Biomaterials 2019, 203, 23–30.
- Zhao, J.; Zhang, Z.; Xue, Y.; Wang, G.; Cheng, Y.; Pan, Y.; Zhao, S.; Hou, Y. Anti-tumor macrophages activated by ferumoxytol combined or surface-functionalized with the TLR3 agonist poly (I: C) promote melanoma regression. Theranostics 2018, 8, 6307–6321.
- Rojas, J.M.; Sanz-Ortega, L.; Mulens-Arias, V.; Gutierrez, L.; Perez-Yague, S.; Barber, D.F. Superparamagnetic iron oxide nanoparticle uptake alters M2 macrophage phenotype, iron metabolism, migration and invasion. Nanomedicine 2016, 12, 1127–1138.
- Kang, H.; Kim, S.; Wong, D.S.H.; Jung, H.J.; Lin, S.; Zou, K.; Li, R.; Li, G.; Dravid, V.P.; Bian, L. Remote Manipulation of Ligand Nano-Oscillations Regulates Adhesion and Polarization of Macrophages in vivo. Nano Lett. 2017, 17, 6415–6427.
- Guo, J.-C.; An, Q.; Guo, M.; Xiao, Y.; Li, B.; Gao, F.; Wang, Y.; Li, J.; Wang, Y.; Liu, Y.; et al. Oxygen-independent free radical generation mediated by core-shell magnetic nanocomposites synergizes with immune checkpoint blockade for effective primary and metastatic tumor treatment. Nano Today 2021, 36, 101024.
- Yang, Y.; Tian, Q.; Wu, S.; Li, Y.; Yang, K.; Yan, Y.; Shang, L.; Li, A.; Zhang, L. Blue light-triggered Fe2+-release from monodispersed ferrihydrite nanoparticles for cancer iron therapy. Biomaterials 2021, 271.
- Chen, L.; Ma, X.; Dang, M.; Dong, H.; Hu, H.; Su, X.; Liu, W.; Wang, Q.; Mou, Y.; Teng, Z. Simultaneous T Cell Activation and Macrophage Polarization to Promote Potent Tumor Suppression by Iron Oxide-Embedded Large-Pore Mesoporous Organosilica Core-Shell Nanospheres. Adv. Healthc. Mater. 2019, 8, e1900039.
- Rao, L.; Zhao, S.K.; Wen, C.; Tian, R.; Lin, L.; Cai, B.; Sun, Y.; Kang, F.; Yang, Z.; He, L.; et al. Activating Macrophage-Mediated Cancer Immunotherapy by Genetically Edited Nanoparticles. Adv. Mater. 2020, 32, e2004853.
- Zhang, W.; Cao, S.; Liang, S.; Tan, C.H.; Luo, B.; Xu, X.; Saw, P.E. Differently Charged Super-Paramagnetic Iron Oxide Nanoparticles Preferentially Induced M1-Like Phenotype of Macrophages. Front. Bioeng. Biotechnol. 2020, 8, 537.
- Li, K.; Lu, L.; Xue, C.; Liu, J.; He, Y.; Zhou, J.; Xia, Z.; Dai, L.; Luo, Z.; Mao, Y.; et al. Polarization of tumor-associated macrophage phenotype via porous hollow iron nanoparticles for tumor immunotherapy in vivo. Nanoscale 2020, 12, 130–144.
- Gong, T.; Dong, Z.; Fu, Y.; Gong, T.; Deng, L.; Zhang, Z. Hyaluronic acid modified doxorubicin loaded Fe3O4 nanoparticles effectively inhibit breast cancer metastasis. J. Mater. Chem. B 2019, 7, 5861–5872.
- Li, C.X.; Zhang, Y.; Dong, X.; Zhang, L.; Liu, M.D.; Li, B.; Zhang, M.K.; Feng, J.; Zhang, X.Z. Artificially Reprogrammed Macrophages as Tumor-Tropic Immunosuppression-Resistant Biologics to Realize Therapeutics Production and Immune Activation. Adv. Mater. 2019, 31, e1807211.
- Sun, W.; Yang, J.; Hou, M.; Xie, S.; Xiong, L.; Li, B.; Zhang, C. A Nano “Immune-Guide” Recruiting Lymphocytes and Modulating the Ratio of Macrophages from Different Origins to Enhance Cancer Immunotherapy. Adv. Funct. Mater. 2021.
- Costa da Silva, M.; Breckwoldt, M.O.; Vinchi, F.; Correia, M.P.; Stojanovic, A.; Thielmann, C.M.; Meister, M.; Muley, T.; Warth, A.; Platten, M.; et al. Iron Induces Anti-tumor Activity in Tumor-Associated Macrophages. Front. Immunol. 2017, 8, 01479.
- Pazar, B.; Ea, H.K.; Narayan, S.; Kolly, L.; Bagnoud, N.; Chobaz, V.; Roger, T.; Liote, F.; So, A.; Busso, N. Basic calcium phosphate crystals induce monocyte/macrophage IL-1beta secretion through the NLRP3 inflammasome in vitro. J. Immunol. 2011, 186, 2495–2502.
- He, X.Y.; Liu, B.Y.; Xu, C.; Zhuo, R.X.; Cheng, S.X. A multi-functional macrophage and tumor targeting gene delivery system for the regulation of macrophage polarity and reversal of cancer immunoresistance. Nanoscale 2018, 10, 15578–15587.
- Jiang, H.; Guo, Y.; Wei, C.; Hu, P.; Shi, J. Nanocatalytic Innate Immunity Activation by Mitochondrial DNA Oxidative Damage for Tumor-Specific Therapy. Adv. Mater. 2021, e2008065.
- Ai, X.; Hu, M.; Wang, Z.; Lyu, L.; Zhang, W.; Li, J.; Yang, H.; Lin, J.; Xing, B. Enhanced Cellular Ablation by Attenuating Hypoxia Status and Reprogramming Tumor-Associated Macrophages via NIR Light-Responsive Upconversion Nanocrystals. Bioconjug. Chem. 2018, 29, 928–938.
- Kang, H.; Zhang, K.; Wong, D.S.H.; Han, F.; Li, B.; Bian, L. Near-infrared light-controlled regulation of intracellular calcium to modulate macrophage polarization. Biomaterials 2018, 178, 681–696.
- Liu, Y.; Wen, Y.; Chen, X.; Zhu, X.; Yu, Q.; Gong, Y.; Yuan, G.; Liu, J.; Qin, X. Inflammation-responsive functional Ru nanoparticles combining a tumor-associated macrophage repolarization strategy with phototherapy for colorectal cancer therapy. J. Mater. Chem. B 2019, 7, 6210–6223.
- Li, K.; Lin, C.; He, Y.; Lu, L.; Xu, K.; Tao, B.; Xia, Z.; Zeng, R.; Mao, Y.; Luo, Z.; et al. Engineering of Cascade-Responsive Nanoplatform to Inhibit Lactate Efflux for Enhanced Tumor Chemo-Immunotherapy. ACS Nano 2020, 14, 14164–14180.
- Leonard, F.; Curtis, L.T.; Ware, M.J.; Nosrat, T.; Liu, X.; Yokoi, K.; Frieboes, H.B.; Godin, B. Macrophage Polarization Contributes to the Anti-Tumoral Efficacy of Mesoporous Nanovectors Loaded with Albumin-Bound Paclitaxel. Front. Immunol. 2017, 8, 693.
- Mocan, T.; Matea, C.; Tabaran, F.; Iancu, C.; Orasan, R.; Mocan, L. In Vitro Administration of Gold Nanoparticles Functionalized with MUC-1 Protein Fragment Generates Anticancer Vaccine Response via Macrophage Activation and Polarization Mechanism. J. Cancer 2015, 6, 583–592.
- Li, D.; Zhang, M.; Xu, F.; Chen, Y.; Chen, B.; Chang, Y.; Zhong, H.; Jin, H.; Huang, Y. Biomimetic albumin-modified gold nanorods for photothermo-chemotherapy and macrophage polarization modulation. Acta Pharm. Sin. B 2018, 8, 74–84.
