Obesity and ageing place a tremendous strain on the global healthcare system. Age-related sarcopenia is characterized by decreased muscular strength, decreased muscle quantity, quality, and decreased functional performance. Sarcopenic obesity (SO) is a condition that combines sarcopenia and obesity and has a substantial influence on the older adults’ health.
- exercise
- sarcopenia
- sarcopenic obesity
- ageing
- skeletal muscle
1. Introduction
Ageing is determined by genetic background and influenced by various environmental factors [1]. When almost every organ in the body is affected by the harmful effects of ageing, the most phenotypically visible changes affect body composition, primarily skeletal muscle, adipose, and bone tissues [2]. Muscle mass constitutes about 42% of body mass in adult humans, but it decreases to about 27% in older people [3]. The loss of body mass becomes noticeable after reaching the age of 50 [4] and further accelerates with ageing. This condition is called sarcopenia (the Greek sárx, “flesh” and peníā” poverty”) [5][5]. Often, but not always, sarcopenia, especially after the age of 60 is accompanied by a significant increase of body fat (sarcopenic obesity). This type of sarcopenia is especially harmful to the human body since it directly decreases human exercise capacity and accelerates the rate of other age-related multi-organ dysfunctions [1]. Physical activity attenuates the rate of the ageing-related deterioration of muscle function, but it cannot stop this process [6].
According to the current definition, sarcopenia is an age-related, progressive, and generalized skeletal muscle disorder characterized by low muscle strength (dynapenia), low muscle quantity and quality, and reduced functional performance [5]. This current definition of sarcopenia, unlike the previous ones, pays more attention to the reduction of muscle strength and physical impairment than just to the loss of muscle mass [5]. Muscle mass decreases with age, mainly at the expense of fast-twitch type II fibres [7]. The loss of muscle mass does not fully explain a parallel decline in muscle function; muscle mass and muscle strength decrease with age, but the decline in strength is 2–5 times faster than predicted from the decrease in muscle mass alone [7].
In addition to their prominent role in motor function, muscles are vital for metabolic homeostasis [8]. Sarcopenia is a crucial component of the frailty syndrome and leads to an increased likelihood of adverse side effects, such as falls, fractures, physical disability, and mortality [2][8][2,8] and a higher risk of insulin resistance, diabetes, and cardiovascular diseases [9][10][11][12][13][14][9-14]. As with muscle tissue, maximum bone mass is reached around age 30, remains constant in young adulthood, and slowly declines with age [15]. In women, after menopause, the rate of bone loss increases, leading to osteoporosis earlier than in men. However, at a later age, the rate of bone loss is similar for both sexes [16].
Unlike muscle and bone tissue, total body fat increases with ageing up to a certain age. Only later in very old age does also the adipose tissue decline [16]. However, a more critical ageing-related transformation is the pronounced redistribution of adipose tissue [5]. A continuing loss of subcutaneous adipose tissue (SAT) with age is accompanied by increased visceral obesity and an accumulation of adipocytes and lipids in different depots such as bone marrow, liver, and particularly skeletal muscle (myosteatosis) [5][17][5,17].
Sarcopenic obesity (SO) occurs when a decrease in lean body mass is accompanied by an excessive accumulation of adipose tissue, especially visceral fat. The risk and incidence of SO increase with age [5]. The increasing incidence of SO and its serious consequences make it a significant health burden in an ageing population due to the frequency of serious complications [18]. Both obesity and sarcopenia are characterized by a subacute, chronic pro-inflammatory state (low-grade inflammation) that affects metabolic processes, disrupting the functioning of both adipose and skeletal muscle tissue [19]. Sarcopenic obesity causes, however, much more severe health consequences than obesity or sarcopenia alone [20]. Regrettably, there is no clear definition of this state [5]. The coexistence of obesity with sarcopenia accelerates muscle mass and function loss, reduces physical performance, and increases mortality risk [20]. Both obesity and the ageing process contribute to the ectopic deposition of adipose tissue in skeletal muscles and other organs [17][18][21][22][17,18,21,22]. In addition to further muscle dysfunctions, this phenomenon contributes to other disorders: oxidative stress, inflammation, mitochondrial dysfunction, and insulin resistance [17][21][17,21]. It is worth noting that the term “osteosarcopenic obesity” has recently been proposed to emphasize the importance of excessive obesity in the deterioration of muscle and bone health [23]. Perna et al. [24] proposed the existence of two phenotypes: osteosarcopenic visceral obesity (OVO) and osteosarcopenic subcutaneous obesity (OSO). They have shown that the visceral obesity form is much more common and that older patients suffering from OVO have a greater risk of fractures, inflammation and metabolic disorders than those with OSO. Moreover, patients with OSO seem to benefit from this type of obesity, in line with the often described "obesity paradox" [24][25][24,25].
2. Pathomechanism of changes in skeletal muscle in sarcopenia and sarcopenic obesity
Sarcopenia is caused by a combination of factors, including neurological factors associated with loss of motor neurons, loss of muscle motor units, endocrine changes, and lifestyle changes associated with sedentary behaviour and poor nutrition [26][27][28][29][26-29] (Figure 1).
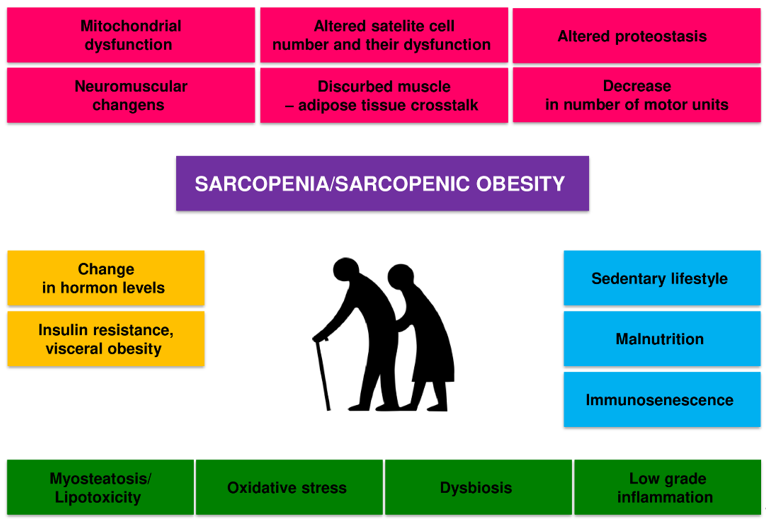
Figure 1. Potential pathogenic mechanisms of age-related sarcopenia and sarcopenic obesity.
There is a dynamic balance between the synthesis and degradation of muscle proteins in the body. Muscle hypertrophy occurs when the synthesis of proteins exceeds their breakdown, and skeletal muscle atrophy occurs when the breakdown is dominant. The mechanisms of sarcopenia and SO development are diverse, complex, and not fully understood. Several factors can influence the development of sarcopenia in the elderly, including hormone and cytokine imbalance, age-associated systemic inflammation (inflammaging), gut microbiota dysbiosis, microcirculation disorders, metabolic disorders, predominantly obesity and insulin resistance [26][27][26,27]. Old age-related physical inactivity and quantitative and qualitative malnutrition will also contribute to this process [28][29][28,29].
All these factors can interact in a complex way on skeletal muscle, reducing the expression of skeletal muscle growth factors and increasing oxidative stress, and the activity of the ubiquitin-proteasome system and autophagy [26]. These mechanisms disrupt the balance between the synthesis and breakdown of muscle proteins, lead to a decrease in the number and function of satellite cells and dysfunction of mitochondria, and ultimately to atrophy and dysfunction of skeletal muscles [26][27][30][26,27,30]. The disorders of the nervous motor system and its interaction with skeletal muscles also play a significant role [31]. The loss of alpha motor neurons and disorders of neuromuscular connections contribute to the disappearance of muscle fibres, especially Type II fibres, and the transition of Type II muscle fibres to Type I muscle fibres [26][27][26,27]. Changes in the structure and function of the neuromuscular junction with ageing also contribute considerably to sarcopenia [32]. In general, it is considered that the main factors that contribute to the loss of muscle power generating capabilities during ageing are as follows: (i) loss of muscle mass, (ii) fast-to-slow transition in areal fibre type composition, (iii) an increase in connective tissue, (iv) altered neural drive (for review see, Degens, 2019) [4].
Maintaining skeletal muscle mass and function is multifaceted and depends on complex regulatory processes in response to ageing, disease and injury, exercise and diet [33]. These processes include the process of myogenesis and, in particular, the activation of satellite muscle cells and proliferation of myoblasts; the withdrawal of myoblasts from the cell cycle, their subsequent differentiation and fusion into multinucleated muscle fibres [34][35][34,35]. They also include the processes of repair and reconstruction of muscle tissue [36][37][36,37] and balance between the breakdown of skeletal muscle proteins and their synthesis [38]. A signalling system including growth factors such as insulin-like growth factor 1 (IGF1) and a cascade of intracellular components plays a vital role in regulating skeletal muscle growth. The Akt kinase, also known as protein kinase B (PKB), is the central component of this cascade, controlling both protein synthesis via the mammalian target of rapamycin (mTOR), also referred to as rapamycin mechanistic target, and glycogen synthase kinase 3 (GSK3), and protein degradation via transcription factors of the FoxO family [39]. Activation of this pathway is essential to induce load-induced skeletal muscle hypertrophy. mTOR is present in at least two multi-protein complexes known as mTORC1 and mTORC2. mTORC1, a raptor-binding protein, can stimulate protein synthesis. Increased protein synthesis and hypertrophy necessitate increased ribosome activity, which can be done via increasing ribosome efficiency (i.e. more mRNA translation to the ribosome) and/or ribosome capacity (through ribosome biogenesis) mTORC1 activity regulates both processes at least partially [39][39]. The sarcopenic muscles have impaired this pathway, which may play a role in the development of sarcopenia [40].
How the size of individual organs of our body is so precisely controlled remains a complex biological problem. During an organism development, all its elements are subject to many changes, including changes in size. Hippo pathway is responsible for the control of the growth processes, partly cell cycle and apoptosis in all eukaryotic cells. This unique pathway integrates signals from many surface receptors and other internal molecular signal to manage regeneration and cell division processes. Briefly, Hippo pathway constitutes of central ‘core kinases’ that interact with specific adhesion molecules via up-stream modulators. The effector part contains a target of the core kinases: transcriptional activator Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ). Hippo signalling down-regulate the proliferation stimuli and loss of MST1/2 activity or overexpression of YAP-1 might result in tissue overgrowth. Moreover, loss of MST1 or MST2 gene function due to mutation leads to an instant raise of mTORC1 (mTOR complexes 1) but not mTORC2 activity, confirming the involvement of both pathways, Hippo and mTOR (mammalian target of rapamycin serine/threonine protein kinase), in the monitoring of cell growth and organ size [41]. The growing volume of experimental evidence indicates that the Hippo pathway might also be responsible for the regulation of physiological and pathological changes taking place in muscle tissue [42][43][42,43].
Muscle stem cells, also known as satellite cells, play a crucial role in muscle fibre regeneration, repair, and muscle hypertrophy. Satellite cells are found under the basal lamina of muscle fibres and are mitotically quiescent in adult life [44]. When a muscle is injured, the dormant satellite cells are activated, which leads to their proliferation and differentiation into myoblasts [44]. With age, the regenerative abilities of ageing muscles gradually deteriorate. The number of satellite muscle cells, especially satellite type II, clearly decreases, and their function is impaired, leading to the accumulation of unrepaired muscle cells [35][44][35,44].
Although the mechanisms underlying sarcopenia and its consequences are still not fully understood, chronic inflammation and immune disorders are essential. Although still not fully defined, the concept of immunosenescence is used to describe the totality of age-related changes leading to the deterioration of the functional state of the immune system [1][45][46][1,45,46]. The ageing process is associated with the remodelling of the immune system, leading to a decrease in immune effectiveness, increased susceptibility to infectious diseases and age-related inflammatory diseases and cancers [46][47][46,47].
In the process of ageing, the immune cell secretion profile is altered, increasing the release of pro-inflammatory cytokines and developing “inflammaging, occurring in the absence of infection (sterile inflammation), leading to tissue damage [19][46][19,46]. The pro-inflammatory cytokines may contribute to the development of sarcopenia by activating the ubiquitin-protease system [48][49][48,49]. They may also antagonise the pro anabolic effects of insulin growth factor-1 (IGF-1) [50][51][50,51]. Inflammaging may also be responsible for anabolic resistance (AR), and the fact that skeletal muscle protein biosynthesis in response to physiological stimuli is insufficient to maintain skeletal muscle in older adults [52].
Chronic low-grade inflammation associated with obesity and the ageing process may affect the simultaneous development of insulin resistance (IR) and AR [19][53][54][55][19,53-55]. The latter is understood as an impaired synthesis of skeletal muscle proteins to anabolic stimuli such as dietary proteins or physical activity [56][57][58][56-58]. Therefore, together, IR and AR can act synergistically, lead to disturbances in adipose tissue metabolism, skeletal muscles and bones, and contribute to the development of type 2 diabetes (T2D) and osteosarcopenic obesity [55][59][55,59]. Both low-grade generalized inflammation and intramuscular fat infiltration can lead to mitochondrial dysfunction and impaired myokine release [60].
As the interaction between the immune cells and skeletal muscles is essential for the proper regeneration of the latter, it is clear that immunosenescence can influence skeletal muscle repair [47]. In injury, immune cells infiltrate skeletal muscle and function by removing necrotic cells and secreting growth factors influencing satellite cell proliferation and differentiation [61]. During ageing, the process of immunosenescence leads to the loss of normal function of these cells and impaired regeneration of skeletal muscles [62].
Interestingly, declining immune function in the elderly is also associated with dysbiosis. A direct link between age-related dysbiosis and age-associated systemic inflammation has been shown in a study in which cohousing germ-free (GF) mice with old, but not young, conventionally raised mice increased intestinal permeability and pro-inflammatory cytokines in the blood leading to age-related inflammation [63].
Although microbes reside in several anatomical locations, colonizing all surfaces covered by epithelia such as the skin, vagina, airways, and mouth, the lower gastrointestinal tract of mammals harbours the greatest density and diversity of commensal microorganisms [64].
Studies conducted over many years have shown that the bacteria living in the alimentary tract have an essential role in food digestion, production of vitamins, a transformation of xenobiotics, promotion of angiogenesis, immunity to infections, and maintenance of immune homeostasis [64]. In addition, the gut microbiota is involved in host metabolism by contributing to bile acid metabolism and recirculation, absorption of iron, magnesium and calcium, and regulation of fat storage [65]. Moreover, it has been shown that gut microbiota is a source of various bacterial products and metabolites that breach the intestinal epithelium. Short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate are widely recognized modulators of immune response in the periphery, produced during bacterial fermentation of indigestible polysaccharides [64].
Interestingly, in the elderly, gut microbiota becomes unstable and less diverse what has been linked with increased frailty and deterioration of the immune system [66]). It is believed that observed in older adults, low gut microbiota richness is a predictor of morbidity and mortality, whereas enrichment of certain bacteria, e.g. Akkermansia and Bifidobacterium is associated with longevity [66].
Age-associated dysbiosis, thining of the mucin layer, and increased epithelial gaps are responsible for increased mucosal barrier permeability, which allows the translocation of microbes and microbial products into the circulation [67]. Animal studies suggest that translocation of microbes and microbial products, termed pathogen-associated molecular patterns (PAMPs), from the gut lumen into the circulation is an important factor contributing to age-associated systemic inflammation and immune system dysregulation involved in numerous age-related diseases in humans [19][63][19,63]. Moreover, it is worth noting that age-associated dysbiosis could promote not only inflammaging but anabolic resistance as well, ultimately conditioning reduced muscle size, impaired muscle function and adverse clinical outcomes [68]. Numerous animal studies show that intestinal microbiota can regulate skeletal muscle function. It is worth noting that GF mice devoid of all microorganisms have lower muscle mass and fewer muscle fibres, whereas muscle atrophy markers are elevated compared to specific pathogen-free (SPF) mice. Observed changes were reversed after faecal microbiota transplantation (FMT) and SCFA supplementation [69].
Further, FMT from elderly (high-functioning group) and (low-functioning group) people into GF mice showed that the grip strength was significantly increased in high-functioning when compared with low-functioning mice [70]. The data of this animal study are supported by a randomized controlled, double-blind study showing that prebiotic supplementation increases the grip strength in older people [71]. The other animal studies show that supplementation with Faecalibacterium prausnitzi increases muscle mass compared to the control group [72].[72]
Moreover, prebiotic (inulin and fructo-oligosaccharides) supplementation increased muscle strength and endurance in older people, suggesting prebiotics' beneficial influence on muscle function. [71]. It is suggested that supplementation with prebiotics increases the abundance of Bifidobacterium and butyrate producers, thereby improving muscle mass and function in older people [73]. A limited number of animal and human studies suggest the existence of the gut-muscle axis actively involved in the pathophysiology of physical frailty and sarcopenia [74].
Factors contributing to age-associated dysbiosis include altered diet, reduced physical activity, pharmaceuticals, altered gut morphology and reduced intestinal functionality [67]. It is still unclear how dysbiosis may contribute to sarcopenia development. It is suggested that dysbiosis affects protein metabolism, including absorption and availability reduction and increased hydrolysis, leading to reduction of muscle protein synthesis and the development of sarcopenia [74]. Moreover, gut microbiota dysbiosis contributes to gut barrier dysfunction facilitating translocation of microbial byproducts, e.g. lipopolysaccharide (LPS), into the circulation, causing systemic low-grade inflammation and insulin resistance and finally leading to sarcopenia [74]. It is also possible that barrier leakiness and microbial dysbiosis in older people could activate immune cells in mucosal tissues, which migrate to the affected organs, e.g. muscles in the periphery [75].
Additionally, gut microbiota dysbiosis results in reduced production of immunoregulatory and anti-inflammatory SCFAs, which could support sarcopenia development. Furthermore, SCFAs affect skeletal muscle cell function by promoting mitochondrial activity [76]. It is postulated that decreased production of SCFAs by age-modified microbiota could promote insulin resistance, decrease mitochondrial fatty acid oxidation, and support intramuscular fatty acid deposition. This leads to decreased muscle strength, insulin resistance, and sarcopenia [68]).
Discussing the role of microbiota products in sarcopenia, we should not forget about toxins having negative effects, such as indoxyl sulfate. It has been observed that circulating levels of microbiota-derived indoxyl sulfate are positively associated with the expression of atroginin-1 and myostatin, which are the main negative regulators of skeletal muscle mass [68]. On the other hand, phenolic compounds produced by gut microbiota can increase glucose uptake in muscle cells, promoting anabolic responses that increase muscle mass [77]. It is worth noting that there is evidence of a connection between microbiota and mitochondrial function. Indeed, decreased butyrate production by dysbiotic gut microbiota impairs mitochondrial function [78].
Furthermore, SCFAs are the putative mediators of the effect of gut microbiota on skeletal muscle by acting on muscle mitochondria [74]. Thus, dysbiosis and reduced production of SCFAs in the elderly may contribute to the development of sarcopenia. Other studies suggest that mitochondrial dysfunction in muscle cells occurs in sarcopenia [79]. A trigger factor of inflammation in sarcopenia could be oxidized cell-free mtDNA from aged mitochondria generated in dysbiotic older people. Oxidized cell-free mtDNA as a damage-associated molecular pattern (DAMP) could activate innate immunity and promote the subsequent synthesis of pro-inflammatory mediators, which fuels sterile inflammation contributing to muscle wasting [79]. Finally, gut microbiota dysbiosis can promote “anorexia of ageing”. Indeed, microbial metabolites can act as endocrine regulators of appetite, as shown in animal models of inflammation induced by Escherichia coli. [80]([80]. This may suggest that, in older people, the dysbiotic gut microbiota could influence the onset of sarcopenia and physical frailty also by promotion of malnutrition [68]). Further work is required to fully understand the role of gut microbiota dysbiosis in sarcopenia development.
At least in part, mitochondrial dysfunction is linked to the ageing and obesity processes [81][82][81,82]. After the deposition of intracellular lipids, mitochondrial dysfunction and increased generation of reactive oxygen species occur in the muscles, disrupting muscle protein synthesis and impairing skeletal muscle function [83][84][83,84].
3. Obesity
Obesity is usually defined as an excessive or abnormal accumulation of body fat that adversely affects health [85]. The adipose tissue dysfunction in the present in obesity leads to low-grade chronic inflammation, characterized by the activation of pro-inflammatory pathways and a shift in adipokine release towards a pro-inflammatory profile [86][87][86,87]. It is associated with developing metabolic and cardiovascular diseases and some cancers [88][89][88,89].
The prevalence of obesity has increased radically recently and continues to rise among the elderly worldwide [90]. The prevalence of obesity increases with age, and in an ageing population, this obesity epidemic is a growing health care problem [91][92][93][91-93].
In mammals, adipose tissue is not homogeneous; there are two main types: white adipose tissue (WAT), which stores excess energy as triglycerides, and brown adipose tissue (BAT), which dissipates stored energy as heat [94][95][94,95]. Studies in recent years have revealed other types of adipose tissue. Of particular interest are newly identified adipocytes displaying features of both brown and white fat cells, usually developing in subcutaneous WAT from a separate subset of preadipocytes [96]. Due to their appearance and location, these adipocytes have been called brite or beige adipocytes
There are two main anatomical compartments in WAT: subcutaneous (SAT) and visceral adipose tissue (VAT), which demonstrate different metabolic and immunological profiles [97][98][97,98]. Both VAT and SAT store energy in the form of triacylglycerols and are endocrine organs that regulate energy homeostasis and metabolism. VAT also provides a protective lining for vital, visceral organs, while the subcutaneous WAT insulation against temperature fluctuations [95]. As opposed to SAT accumulation, high VAT is associated with increased metabolic and cardiovascular diseases and premature death risk [88][99][88,99].
It is now recognized that the development of obesity is associated with alterations in gut microbial composition. Microorganisms colonize all surfaces covered by epithelia and they occur in the greatest number in the alimentary tract.
3.1. Adipokines
Presently, it has become clear that, in addition to their role in energy storage and adaptive thermogenesis, white (WAT) and brown (BAT) adipose tissues are endocrine organs. WAT and BAT communicate with other organs to regulate metabolism by secreting adipokines and batokines, respectively, signalling types of lipids (lipokines) and exosomal microRNAs (miRNAs) [100][101][100,101]. Especially white adipose tissue is a hormonal organ that produces biologically active adipokines, such as adiponectin (APN), IL-1, IL-6, IL-8, IFN-γ, TNF-α, leptin apelin, chemerin, and resistin. Adipokines can regulate metabolic homeostasis and influence immune function [102].
Subjects with SO have elevated plasma levels of pro-inflammatory adipokines [20], which are inversely correlated with muscle strength in these people [20][103][20,103]. These substances also suppressed muscle regeneration and promoted atrophy [104][105].[104,105]
3.2. Myosteatosis
Fats can build up in the muscle fibres themselves, called intramuscular fat (IMC), but also between skeletal muscle bundles and below the muscle fascia, called intermuscular fat (IMAT) [22][106][22,106]. Fat infiltration (myosteatosis) contributes significantly to the deterioration of muscle function with age [106]. Increased IMAT leads to impaired contractility of skeletal muscles and their metabolic function [107]. Myosteatosis leads to metabolic dysfunction via lipotoxicity and insulin resistance. Furthermore, it has been associated with inflammation and could damage muscle function and quality [22][106][108][22,106,108]. It was recently shown that inhibition of Yap impairs fatty acid oxidation and leads to lipotoxicity in skeletal muscle [109]. Type I fibres (slow-twitch oxidation fibres) collect more lipids with age in humans than type II fibres [110]. The accumulation of adipose tissue in the skeletal muscles can support the conversion of type II fibres to type I and reduce skeletal muscle strength [111].
It should also be noted that intramuscular fat also can secrete pro-inflammatory adipokines, contributing to systemic inflammation and affecting skeletal muscle metabolism [60][112][60,112]. It has also been suggested that muscle stem cells may be one of the factors responsible for the accumulation of adipocytes. A type of stem cell other than the satellite cell population has been described. Those cells known as fibro/adipogenic progenitors (FAPs) or mesenchymal interstitial cells are multi-potent progenitors and can differentiate, under certain conditions, such as muscle damage, unlike satellite cells not into myoblasts but adipocytes [113]. FAPs are critical regulators of muscle regeneration, but in pathological situations, such as obesity, they can cause chronic inflammation, fibrosis, and intramuscular fat accumulation in skeletal muscle [113][114][113,114]. In obesity, adipokines released mainly from visceral WAT increase FAP adipogenesis, while substances released from myofibers inhibit it [114].
4. Physical exercise as a method of preventing sarcopenia
The primary method of preventing and inhibiting the progression of age-related sarcopenia and SO is physical activity [115][116][117][118][115-118]. Lack of physical activity in old age is an important risk factor for sarcopenia [115]. Nevertheless, the mechanisms by which exercise can slow down sarcopenia and obesity are complex (for review see, Lazarus and Harridge, 2017; Degens, 2019) [4][6][4,6]. Exercise is critical for maintaining a healthy energy balance and combined with a low-calorie diet, exercise-related energy expenditure can result in a negative energy balance. Exercise is a potent anabolic stimulus and also can improve muscle strength, gait, balance, and aerobic capacity [115][116][117][118][115-118].
Resistance training is considered an important strategy to counter sarcopenia; they promote satellite cells activation and proliferation and enhance muscle protein synthesis while inhibiting their breakdown, resulting in increased skeletal muscle mass and strength [119]. Resistance exercise promotes mTOR signalling, which is responsible for the changes in protein synthesis, autophagy, and expression of peroxisome proliferator alpha co-activator 1 (PGC-1) ribosome biogenesis that this exercise elicit. It has been known that the Hippo signalling pathway, particularly YAP protein, participates in response to and conducting mechanical stimuli. To well-known and scientifically documented cellular signal transduction systems involved in the regulation of muscle hypertrophy (IGF-1-PI3K-Akt-mTOR) and their atrophy (myostatin-Smad3) one can add a Hippo pathway as an important additional mediator of balance between development and differentiation or atrophy and muscular tissue decay as it has been shown above.
Systematic resistance exercises increase the size of muscle fibres, especially fast-twitch fibres [120]. Resistance exercise is widely recommended to improve muscle mass and skeletal muscle function in the elderly [115][116][117][120][115-117,120]. Increasing the intensity of resistance training and involving larger muscle groups appears to yield more significant effects [117][118][121][117,118,121]. As skeletal muscle hypertrophic potential decreases in old age, it is recommended that patients begin resistance exercises as early as possible [121][122][121,122]. Resistance exercises, despite their benefits, have some drawbacks: they can increase the risk of injury, and the range of repetitions can cause boredom and increase the risk of quitting training [118]. Resistance exercise's impact on body composition and skeletal muscle function in older persons with sarcopenic obesity has received relatively little attention [123]. Despite this, most published evidence indicates that resistance exercise effectively increases body composition, muscle strength, and physical performance in these individuals [124][125][126][124-126].
The primary purpose of aerobic exercise in the elderly is to increase/maintain the aerobic capacity of their skeletal muscles [127]. Low – moderate-intensity physical activities (so-called: aerobic exercises) in general have a far lesser effect on the increases of muscle mass than resistance training, but first of all, it is potent to enhance cardio-vascular heath, muscle oxidative capacity, exerts an anti-inflammatory effect, reduces oxidative stress and insulin resistance [128][129][128,129] and plays an important role in the control of body mass. They may also inhibit the release of myostatin (MSTN) [107][130][107,130]. In addition, aerobic training may have a beneficial effect on maintaining the correct mass of adipose tissue and counteract the development of obesity [131]. Chen et al. [124] demonstrated that aerobic training significantly reduced total fat and visceral adipose tissue in subjects with SO. Interestingly, a more substantial effect was observed after the combination of aerobics and resistance training.
The theoretical foundation for mixing resistance training, walking, aerobic training, balance training, and other types of training in multimodal exercise therapy is well-founded. [132]. Although still in their infancy, existing research appears to back up these assertions [117][132][117,132].
Alternative approaches are offered since some elderly persons are unable to exercise for various reasons. Preliminary findings suggest that whole-body vibration (WBV) and whole-body electromyostimulation (WB-EMS) may be effective in the treatment of sarcopenia, but more research is needed. [133][134][133,134].
A new blood flow restriction (BFR) approach, which partially restricts arterial inflow while totally limiting venous outflow in the muscles during exercise, offers an intriguing alternative [135][136][135,136]. During low-intensity training, this approach allows for a significant gain in skeletal muscle strength. [135]. However, some experts are concerned about the method's potential adverse side effects and propose that training only occurs under-qualified staff's supervision [135][136][135,136].
Although physical activity plays an important role in slowing down the process of sarcopenia in ageing people, even a high dose of physical activity can not stop the ageing-related loss of muscle mass, their force and power generating capabilities in humans (see e.g. Lazarus and Harridge, 2017) [6].
Research has consistently shown that regular exercise provides remarkable health benefits, plays a role in preventing or reducing the effects of chronic disease, slowing biological ageing, and prolonging life [137]. The mechanisms of these health benefits are complex but can be at least partly attributed to bioactive substances released into the circulation during exercise [138][139][138,139]. It is now widely accepted that skeletal muscles, besides their primary functions, play the role of endocrine organs, producing and releasing cytokines and other peptides, exerting autocrine, paracrine and hormonal effects on various tissues [138].
Disorders in myokine secretion may play a role in the pathogenesis of age-related and metabolic diseases, including obesity, T2D, sarcopenia, and SO [140][141][142][140-142]. Ageing leads to a decrease in the secretion of most myokines, including apelin, BAIBA, decorin, IGF-1, IL-15, irisin, sesterin, SPARC, while the secretion of myostatin increases. These processes were partially reversed by regular physical activity [143] (Figure. 2).
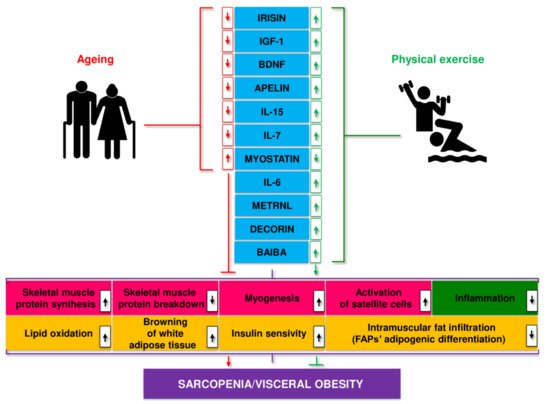
Figure 2. Myokines linked to age-related changes, their release during exercise, and putative mechanisms of action.
Muscle hypertrophy is a critical adaptation to regular exercise, particularly resistance training. This effect is likely mediated by insulin-like growth factor 1 (IGF-1) generated in muscles during exercise [39]. Other myokines produced by exercise also appear to positively affect the proliferation of satellite cells and muscle hypertrophy [144][145][144,145]. Reduced myostatin levels, a muscle growth inhibitor, and altered control of myostatin activity in exercising skeletal muscles, presumably influenced by other myokines, may also contribute to muscle hypertrophy. Research in recent years suggests that myokines may act as diagnostic biomarkers and therapeutic targets in sarcopenia and SO [142][143][142,143].
5. Conclusions
Obesity and ageing are major health costs for the world's adult population. Both factors increase the risk of developing related metabolic disorders. Sarcopenic obesity is defined by the presence of both sarcopenia and obesity. It has a significant impact on the health of the elderly. Visceral adipose tissue (VAT) becomes dysfunctional during obesity and ageing and plays an essential role in its pathophysiology. Changes in VAT related to obesity and ageing are partly due to chronic local inflammation. The gut-muscular axis may be involved in the pathogenesis of sarcopenia and SO. Recent research supports the notion that an unhealthy microbiota may play a role in the onset and progression of sarcopenia and SO. Due to its complex pathophysiology and limitations, there is disagreement and difficulty in defining, diagnosing, and treating SO. Numerous studies have shown that myokines, released by skeletal muscles, play a vital role in controlling muscle hypertrophy, function, and metabolic balance. Myokine dysfunction can trigger and exacerbate the pathogenesis of underlying metabolic and age-related disorders, such as obesity, sarcopenia, type 2 diabetes (T2D), and SO. Physical activity and proper nutritional supplementation are the only practical approaches to delay the onset and treat sarcopenia, especially sarcopenic obesity. Although physical activity cannot fully inhibit the process of sarcopenia and the ageing-related deterioration of muscle function, it can delay the onset of sarcopenia and attenuate its rate. This is why physical training involving both resistance and endurance training in appropriate dose are highly recommended to practice even at an advanced age.
References
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front Med (Lausanne) 2018, 5, 61, doi:10.3389/fmed.2018.00061.
- Duda, K.; Majerczak, J.; Nieckarz, Z.; Heymsfield, S.B.; Zoladz, J.A. Human body composition and muscle mass. In Muscle and Exercise Physiology, Zoladz, J.A., Ed. Academic Press: London, 2019; pp. 3-26.
- Lee, R.C.; Wang, Z.M.; Heymsfield, S.B. Skeletal muscle mass and aging: regional and whole-body measurement methods. Can J Appl Physiol 2001, 26, 102-122, doi:10.1139/h01-008.
- Degens, H. Human ageing: impact on muscle force and power. In Muscle and exercise physiology, Zoladz, J.A., Ed. .Academic Press: London 2019; pp. 423-432.
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16-31, doi:10.1093/ageing/afy169.
- Lazarus, N.R.; Harridge, S.D.R. Declining performance of master athletes: silhouettes of the trajectory of healthy human ageing? J Physiol 2017, 595, 2941-2948, doi:10.1113/JP272443.
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 2012, 3, 260, doi:10.3389/fphys.2012.00260.
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Joint Bone Spine 2019, 86, 309-314, doi:10.1016/j.jbspin.2018.08.001.
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes 2019, 12, 1057-1072, doi:10.2147/DMSO.S186600.
- Hong, S.-h.; Choi, K.M. Sarcopenic obesity, insulin resistance, and their implications in cardiovascular and metabolic consequences. International journal of molecular sciences 2020, 21, 494.
- Poggiogalle, E.; Mendes, I.; Ong, B.; Prado, C.M.; Mocciaro, G.; Mazidi, M.; Lubrano, C.; Lenzi, A.; Donini, L.M.; Siervo, M. Sarcopenic obesity and insulin resistance: Application of novel body composition models. Nutrition 2020, 75-76, 110765, doi:10.1016/j.nut.2020.110765.
- Xia, M.F.; Chen, L.Y.; Wu, L.; Ma, H.; Li, X.M.; Li, Q.; Aleteng, Q.; Hu, Y.; He, W.Y.; Gao, J., et al. Sarcopenia, sarcopenic overweight/obesity and risk of cardiovascular disease and cardiac arrhythmia: A cross-sectional study. Clin Nutr 2021, 40, 571-580, doi:10.1016/j.clnu.2020.06.003.
- Kang, D.O.; Park, S.Y.; Choi, B.G.; Na, J.O.; Choi, C.U.; Kim, E.J.; Rha, S.W.; Park, C.G.; Hong, S.J.; Seo, H.S. Prognostic Impact of Low Skeletal Muscle Mass on Major Adverse Cardiovascular Events in Coronary Artery Disease: A Propensity Score-Matched Analysis of a Single Center All-Comer Cohort. J Clin Med 2019, 8, 712, doi:10.3390/jcm8050712.
- Pacifico, J.; Geerlings, M.A.; Reijnierse, E.M.; Phassouliotis, C.; Lim, W.K.; Maier, A.B. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Experimental Gerontology 2020, 131, 110801.
- Matkovic, V.; Jelic, T.; Wardlaw, G.M.; Ilich, J.Z.; Goel, P.K.; Wright, J.K.; Andon, M.B.; Smith, K.T.; Heaney, R.P. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest 1994, 93, 799-808, doi:10.1172/JCI117034.
- Kelly, T.L.; Wilson, K.E.; Heymsfield, S.B. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One 2009, 4, e7038, doi:10.1371/journal.pone.0007038.
- Zamboni, M.; Gattazzo, S.; Rossi, A.P. Myosteatosis: a relevant, yet poorly explored element of sarcopenia. Eur Geriatr Med 2019, 10, 5-6, doi:10.1007/s41999-018-0134-3.
- Polyzos, S.A.; Margioris, A.N. Sarcopenic obesity. Hormones (Athens) 2018, 17, 321-331, doi:10.1007/s42000-018-0049-x.
- Ferrucci, L.; Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018, 15, 505-522, doi:10.1038/s41569-018-0064-2.
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 2018, 14, 513-537, doi:10.1038/s41574-018-0062-9.
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest 2017, 127, 43-54, doi:10.1172/JCI88880.
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front Endocrinol (Lausanne) 2016, 7, 69, doi:10.3389/fendo.2016.00069.
- Ilich, J.Z.; Kelly, O.J.; Inglis, J.E.; Panton, L.B.; Duque, G.; Ormsbee, M.J. Interrelationship among muscle, fat, and bone: connecting the dots on cellular, hormonal, and whole body levels. Ageing research reviews 2014, 15, 51-60.
- Perna, S.; Spadaccini, D.; Nichetti, M.; Avanzato, I.; Faliva, M.A.; Rondanelli, M. Osteosarcopenic Visceral Obesity and Osteosarcopenic Subcutaneous Obesity, Two New Phenotypes of Sarcopenia: Prevalence, Metabolic Profile, and Risk Factors. J Aging Res 2018, 2018, 6147426, doi:10.1155/2018/6147426.
- Bosello, O.; Vanzo, A. Obesity paradox and aging. Eat Weight Disord 2021, 26, 27-35, doi:10.1007/s40519-019-00815-4.
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol Rev 2019, 99, 427-511, doi:10.1152/physrev.00061.2017.
- Gustafsson, T.; Ulfhake, B. Sarcopenia: What Is the Origin of This Aging-Induced Disorder? Front Genet 2021, 12, 688526, doi:10.3389/fgene.2021.688526.
- Wickramasinghe, K.; Mathers, J.C.; Wopereis, S.; Marsman, D.S.; Griffiths, J.C. From lifespan to healthspan: the role of nutrition in healthy ageing. J Nutr Sci 2020, 9, e33, doi:10.1017/jns.2020.26.
- Murphy, C.H.; Roche, H. Nutrition and physical activity countermeasures for sarcopenia: Time to get personal? Nutrition Bulletin 2018, 43, 374-387.
- Kitada, M.; Koya, D. Autophagy in metabolic disease and ageing. Nat Rev Endocrinol 2021, 17, 647-661, doi:10.1038/s41574-021-00551-9.
- Wilkinson, D.J.; Piasecki, M.; Atherton, P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev 2018, 47, 123-132, doi:10.1016/j.arr.2018.07.005.
- Deschenes, M.R.; Oh, J.; Tufts, H. The role of the neuromuscular junction in sarcopenia. In Sarcopenia, Sakuma, K., Ed. Elsevier: 2021; 10.1016/b978-0-12-822146-4.00010-7pp. 59-80.
- McCormick, R.; Vasilaki, A. Age-related changes in skeletal muscle: changes to life-style as a therapy. Biogerontology 2018, 19, 519-536.
- Le Grand, F.; Rudnicki, M.A. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol 2007, 19, 628-633, doi:10.1016/j.ceb.2007.09.012.
- Yamakawa, H.; Kusumoto, D.; Hashimoto, H.; Yuasa, S. Stem Cell Aging in Skeletal Muscle Regeneration and Disease. Int J Mol Sci 2020, 21, 1830, doi:10.3390/ijms21051830.
- Carosio, S.; Berardinelli, M.G.; Aucello, M.; Musaro, A. Impact of ageing on muscle cell regeneration. Ageing Res Rev 2011, 10, 35-42, doi:10.1016/j.arr.2009.08.001.
- Wall, B.T.; Gorissen, S.H.; Pennings, B.; Koopman, R.; Groen, B.B.; Verdijk, L.B.; van Loon, L.J. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PloS one 2015, 10, e0140903.
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the'anabolic resistance'of ageing. Nutrition & metabolism 2011, 8, 1-11.
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970, doi:10.3390/cells9091970.
- Sakuma, K.; Aoi, W.; Yamaguchi, A. Molecular mechanism of sarcopenia and cachexia: recent research advances. Pflugers Arch 2017, 469, 573-591, doi:10.1007/s00424-016-1933-3.
- Zhou, D.; Conrad, C.; Xia, F.; Park, J.S.; Payer, B.; Yin, Y.; Lauwers, G.Y.; Thasler, W.; Lee, J.T.; Avruch, J., et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 2009, 16, 425-438, doi:10.1016/j.ccr.2009.09.026.
- Watt, K.I.; Judson, R.; Medlow, P.; Reid, K.; Kurth, T.B.; Burniston, J.G.; Ratkevicius, A.; De Bari, C.; Wackerhage, H. Yap is a novel regulator of C2C12 myogenesis. Biochem Biophys Res Commun 2010, 393, 619-624, doi:10.1016/j.bbrc.2010.02.034.
- Sudol, M.; Chen, H.I.; Bougeret, C.; Einbond, A.; Bork, P. Characterization of a novel protein-binding module--the WW domain. FEBS Lett 1995, 369, 67-71, doi:10.1016/0014-5793(95)00550-s.
- Sousa-Victor, P.; Garcia-Prat, L.; Munoz-Canoves, P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat Rev Mol Cell Biol 2021, 10.1038/s41580-021-00421-2, doi:10.1038/s41580-021-00421-2.
- Ray, D.; Yung, R. Immune senescence, epigenetics and autoimmunity. Clin Immunol 2018, 196, 59-63, doi:10.1016/j.clim.2018.04.002.
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 2014, 69 Suppl 1, S4-9, doi:10.1093/gerona/glu057.
- Barberi, L.; Scicchitano, B.M.; De Rossi, M.; Bigot, A.; Duguez, S.; Wielgosik, A.; Stewart, C.; McPhee, J.; Conte, M.; Narici, M., et al. Age-dependent alteration in muscle regeneration: the critical role of tissue niche. Biogerontology 2013, 14, 273-292, doi:10.1007/s10522-013-9429-4.
- Mitch, W.E.; Goldberg, A.L. Mechanisms of muscle wasting—the role of the ubiquitin–proteasome pathway. New England journal of medicine 1996, 335, 1897-1905.
- Ferrucci, L.; Harris, T.B.; Guralnik, J.M.; Tracy, R.P.; Corti, M.C.; Cohen, H.J.; Penninx, B.; Pahor, M.; Wallace, R.; Havlik, R.J. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 1999, 47, 639-646, doi:10.1111/j.1532-5415.1999.tb01583.x.
- Lang, C.H.; Frost, R.A.; Vary, T.C. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab 2007, 293, E453-459, doi:10.1152/ajpendo.00204.2007.
- Frost, R.A.; Lang, C.H. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol (1985) 2007, 103, 378-387, doi:10.1152/japplphysiol.00089.2007.
- Haran, P.H.; Rivas, D.A.; Fielding, R.A. Role and potential mechanisms of anabolic resistance in sarcopenia. J Cachexia Sarcopenia Muscle 2012, 3, 157-162, doi:10.1007/s13539-012-0068-4.
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front Physiol 2017, 8, 1045, doi:10.3389/fphys.2017.01045.
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10-15, doi:10.1016/j.maturitas.2016.11.006.
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol 2016, 229, R67-R81.
- Beals, J.W.; Burd, N.A.; Moore, D.R.; van Vliet, S. Obesity Alters the Muscle Protein Synthetic Response to Nutrition and Exercise. Front Nutr 2019, 6, 87, doi:10.3389/fnut.2019.00087.
- Moore, D.R.; Churchward-Venne, T.A.; Witard, O.; Breen, L.; Burd, N.A.; Tipton, K.D.; Phillips, S.M. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 2015, 70, 57-62, doi:10.1093/gerona/glu103.
- Kumar, V.; Atherton, P.J.; Selby, A.; Rankin, D.; Williams, J.; Smith, K.; Hiscock, N.; Rennie, M.J. Muscle protein synthetic responses to exercise: effects of age, volume, and intensity. J Gerontol A Biol Sci Med Sci 2012, 67, 1170-1177, doi:10.1093/gerona/gls141.
- Meex, R.C.; Blaak, E.E.; van Loon, L.J. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obesity Reviews 2019, 20, 1205-1217.
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev 2017, 35, 200-221, doi:10.1016/j.arr.2016.09.008.
- Saini, J.; McPhee, J.S.; Al-Dabbagh, S.; Stewart, C.E.; Al-Shanti, N. Regenerative function of immune system: Modulation of muscle stem cells. Ageing Res Rev 2016, 27, 67-76, doi:10.1016/j.arr.2016.03.006.
- Domingues-Faria, C.; Vasson, M.P.; Goncalves-Mendes, N.; Boirie, Y.; Walrand, S. Skeletal muscle regeneration and impact of aging and nutrition. Ageing Res Rev 2016, 26, 22-36, doi:10.1016/j.arr.2015.12.004.
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P., et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2018, 23, 570, doi:10.1016/j.chom.2018.03.006.
- Strzepa, A.; Lobo, F.M.; Majewska-Szczepanik, M.; Szczepanik, M. Antibiotics and autoimmune and allergy diseases: Causative factor or treatment? Int Immunopharmacol 2018, 65, 328-341, doi:10.1016/j.intimp.2018.10.021.
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242-249.
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J Exp Med 2019, 216, 20-40, doi:10.1084/jem.20180448.
- Conway, J.; N, A.D. Ageing of the gut microbiome: Potential influences on immune senescence and inflammageing. Ageing Res Rev 2021, 68, 101323, doi:10.1016/j.arr.2021.101323.
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633, doi:10.3390/nu11071633.
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H., et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med 2019, 11, doi:10.1126/scitranslmed.aan5662.
- Fielding, R.A.; Reeves, A.R.; Jasuja, R.; Liu, C.; Barrett, B.B.; Lustgarten, M.S. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Experimental gerontology 2019, 127, 110722.
- Buigues, C.; Fernandez-Garrido, J.; Pruimboom, L.; Hoogland, A.J.; Navarro-Martinez, R.; Martinez-Martinez, M.; Verdejo, Y.; Mascaros, M.C.; Peris, C.; Cauli, O. Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial. Int J Mol Sci 2016, 17, 932, doi:10.3390/ijms17060932.
- Munukka, E.; Rintala, A.; Toivonen, R.; Nylund, M.; Yang, B.; Takanen, A.; Hanninen, A.; Vuopio, J.; Huovinen, P.; Jalkanen, S., et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J 2017, 11, 1667-1679, doi:10.1038/ismej.2017.24.
- Vulevic, J.; Drakoularakou, A.; Yaqoob, P.; Tzortzis, G.; Gibson, G.R. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr 2008, 88, 1438-1446, doi:10.3945/ajcn.2008.26242.
- Liao, X.; Wu, M.; Hao, Y.; Deng, H. Exploring the Preventive Effect and Mechanism of Senile Sarcopenia Based on "Gut-Muscle Axis". Front Bioeng Biotechnol 2020, 8, 590869, doi:10.3389/fbioe.2020.590869.
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol 2021, 21, 739-751, doi:10.1038/s41577-021-00538-7.
- Saint-Georges-Chaumet, Y.; Edeas, M. Microbiota-mitochondria inter-talk: consequence for microbiota-host interaction. Pathog Dis 2016, 74, ftv096, doi:10.1093/femspd/ftv096.
- Houghton, M.J.; Kerimi, A.; Mouly, V.; Tumova, S.; Williamson, G. Gut microbiome catabolites as novel modulators of muscle cell glucose metabolism. FASEB J 2019, 33, 1887-1898, doi:10.1096/fj.201801209R.
- Mottawea, W.; Chiang, C.K.; Muhlbauer, M.; Starr, A.E.; Butcher, J.; Abujamel, T.; Deeke, S.A.; Brandel, A.; Zhou, H.; Shokralla, S., et al. Altered intestinal microbiota-host mitochondria crosstalk in new onset Crohn's disease. Nat Commun 2016, 7, 13419, doi:10.1038/ncomms13419.
- Picca, A.; Fanelli, F.; Calvani, R.; Mule, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut Dysbiosis and Muscle Aging: Searching for Novel Targets against Sarcopenia. Mediators Inflamm 2018, 2018, 7026198, doi:10.1155/2018/7026198.
- Fetissov, S.O. Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour. Nat Rev Endocrinol 2017, 13, 11-25, doi:10.1038/nrendo.2016.150.
- Shou, J.; Chen, P.J.; Xiao, W.H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol Metab Syndr 2020, 12, 14, doi:10.1186/s13098-020-0523-x.
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu Rev Physiol 2019, 81, 19-41, doi:10.1146/annurev-physiol-020518-114310.
- Aon, M.A.; Bhatt, N.; Cortassa, S.C. Mitochondrial and cellular mechanisms for managing lipid excess. Front Physiol 2014, 5, 282, doi:10.3389/fphys.2014.00282.
- Lipina, C.; Hundal, H.S. Lipid modulation of skeletal muscle mass and function. J Cachexia Sarcopenia Muscle 2017, 8, 190-201, doi:10.1002/jcsm.12144.
- Organization, W.H. Obesity: preventing and managing the global epidemic. 2000.
- Mathis, D. Immunological goings-on in visceral adipose tissue. Cell Metab 2013, 17, 851-859, doi:10.1016/j.cmet.2013.05.008.
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A., et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009, 360, 1509-1517, doi:10.1056/NEJMoa0810780.
- Ellulu, M.S.; Patimah, I.; Khaza'ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci 2017, 13, 851-863, doi:10.5114/aoms.2016.58928.
- James, P.T.; Leach, R.; Kalamara, E.; Shayeghi, M. The worldwide obesity epidemic. Obes Res 2001, 9 Suppl 4, 228S-233S, doi:10.1038/oby.2001.123.
- Collaboration, N.R.F. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19· 2 million participants. The Lancet 2016, 387, 1377-1396.
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673-689, doi:10.1007/s40273-014-0243-x.
- Roubenoff, R. Sarcopenic obesity: the confluence of two epidemics. Obes Res 2004, 12, 887-888, doi:10.1038/oby.2004.107.
- Zamboni, M.; Mazzali, G.; Fantin, F.; Rossi, A.; Di Francesco, V. Sarcopenic obesity: a new category of obesity in the elderly. Nutrition, Metabolism and Cardiovascular Diseases 2008, 18, 388-395.
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med 2020, 7, 22, doi:10.3389/fcvm.2020.00022.
- Lanthier, N.; Leclercq, I.A. Adipose tissues as endocrine target organs. Best Pract Res Clin Gastroenterol 2014, 28, 545-558, doi:10.1016/j.bpg.2014.07.002.
- Wu, J.; Bostrom, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G., et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366-376, doi:10.1016/j.cell.2012.05.016.
- Kredel, L.I.; Siegmund, B. Adipose-tissue and intestinal inflammation - visceral obesity and creeping fat. Front Immunol 2014, 5, 462, doi:10.3389/fimmu.2014.00462.
- Tchkonia, T.; Thomou, T.; Zhu, Y.; Karagiannides, I.; Pothoulakis, C.; Jensen, M.D.; Kirkland, J.L. Mechanisms and Metabolic Implications of Regional Differences among Fat Depots. Cell Metab 2013, 17, 644-656, doi:S1550-4131(13)00111-3 [pii];10.1016/j.cmet.2013.03.008 [doi].
- Nam, S.Y. Obesity-Related Digestive Diseases and Their Pathophysiology. Gut Liver 2017, 11, 323-334, doi:10.5009/gnl15557.
- Goody, D.; Pfeifer, A. MicroRNAs in brown and beige fat. Biochim Biophys Acta Mol Cell Biol Lipids 2019, 1864, 29-36, doi:10.1016/j.bbalip.2018.05.003.
- Lee, M.W.; Lee, M.; Oh, K.J. Adipose Tissue-Derived Signatures for Obesity and Type 2 Diabetes: Adipokines, Batokines and MicroRNAs. J Clin Med 2019, 8, 854, doi:10.3390/jcm8060854.
- Weidinger, C.; Ziegler, J.F.; Letizia, M.; Schmidt, F.; Siegmund, B. Adipokines and their role in intestinal inflammation. Frontiers in immunology 2018, 9, 1974.
- Bucci, L.; Yani, S.L.; Fabbri, C.; Bijlsma, A.Y.; Maier, A.B.; Meskers, C.G.; Narici, M.V.; Jones, D.A.; McPhee, J.S.; Seppet, E., et al. Circulating levels of adipokines and IGF-1 are associated with skeletal muscle strength of young and old healthy subjects. Biogerontology 2013, 14, 261-272, doi:10.1007/s10522-013-9428-5.
- Akhmedov, D.; Berdeaux, R. The effects of obesity on skeletal muscle regeneration. Front Physiol 2013, 4, 371, doi:10.3389/fphys.2013.00371.
- Adams, V.; Mangner, N.; Gasch, A.; Krohne, C.; Gielen, S.; Hirner, S.; Thierse, H.-J.; Witt, C.C.; Linke, A.; Schuler, G. Induction of MuRF1 is essential for TNF-α-induced loss of muscle function in mice. Journal of molecular biology 2008, 384, 48-59.
- Waters, D.L. Intermuscular Adipose Tissue: A Brief Review of Etiology, Association With Physical Function and Weight Loss in Older Adults. Ann Geriatr Med Res 2019, 23, 3-8, doi:10.4235/agmr.19.0001.
- Konopka, A.R.; Wolff, C.A.; Suer, M.K.; Harber, M.P. Relationship between intermuscular adipose tissue infiltration and myostatin before and after aerobic exercise training. Am J Physiol Regul Integr Comp Physiol 2018, 315, R461-R468, doi:10.1152/ajpregu.00030.2018.
- Rivas, D.A.; McDonald, D.J.; Rice, N.P.; Haran, P.H.; Dolnikowski, G.G.; Fielding, R.A. Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging but not obesity. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2016, 310, R561-R569.
- Watt, K.I.; Henstridge, D.C.; Ziemann, M.; Sim, C.B.; Montgomery, M.K.; Samocha-Bonet, D.; Parker, B.L.; Dodd, G.T.; Bond, S.T.; Salmi, T.M., et al. Yap regulates skeletal muscle fatty acid oxidation and adiposity in metabolic disease. Nat Commun 2021, 12, 2887, doi:10.1038/s41467-021-23240-7.
- Gueugneau, M.; Coudy-Gandilhon, C.; Theron, L.; Meunier, B.; Barboiron, C.; Combaret, L.; Taillandier, D.; Polge, C.; Attaix, D.; Picard, B., et al. Skeletal muscle lipid content and oxidative activity in relation to muscle fiber type in aging and metabolic syndrome. J Gerontol A Biol Sci Med Sci 2015, 70, 566-576, doi:10.1093/gerona/glu086.
- Mastrocola, R.; Collino, M.; Nigro, D.; Chiazza, F.; D'Antona, G.; Aragno, M.; Minetto, M.A. Accumulation of advanced glycation end-products and activation of the SCAP/SREBP Lipogenetic pathway occur in diet-induced obese mouse skeletal muscle. PLoS One 2015, 10, e0119587, doi:10.1371/journal.pone.0119587.
- Moratal, C.; Raffort, J.; Arrighi, N.; Rekima, S.; Schaub, S.; Dechesne, C.; Chinetti, G.; Dani, C. IL-1β-and IL-4-polarized macrophages have opposite effects on adipogenesis of intramuscular fibro-adipogenic progenitors in humans. Scientific reports 2018, 8, 1-13.
- Biferali, B.; Proietti, D.; Mozzetta, C.; Madaro, L. Fibro-Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Front Physiol 2019, 10, 1074, doi:10.3389/fphys.2019.01074.
- Collao, N.; Farup, J.; De Lisio, M. Role of Metabolic Stress and Exercise in Regulating Fibro/Adipogenic Progenitors. Front Cell Dev Biol 2020, 8, 9, doi:10.3389/fcell.2020.00009.
- Musumeci, G. Sarcopenia and exercise “The State of the Art”. Journal of Functional Morphology and Kinesiology 2017, 2, 40.
- Vlietstra, L.; Hendrickx, W.; Waters, D.L. Exercise interventions in healthy older adults with sarcopenia: A systematic review and meta-analysis. Australas J Ageing 2018, 37, 169-183, doi:10.1111/ajag.12521.
- Beckwee, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J Nutr Health Aging 2019, 23, 494-502, doi:10.1007/s12603-019-1196-8.
- Yoo, S.Z.; No, M.H.; Heo, J.W.; Park, D.H.; Kang, J.H.; Kim, S.H.; Kwak, H.B. Role of exercise in age-related sarcopenia. J Exerc Rehabil 2018, 14, 551-558, doi:10.12965/jer.1836268.134.
- Johnston, A.P.; De Lisio, M.; Parise, G. Resistance training, sarcopenia, and the mitochondrial theory of aging. Appl Physiol Nutr Metab 2008, 33, 191-199, doi:10.1139/H07-141.
- Heo, J.-W.; No, M.-H.; Min, D.-H.; Kang, J.-H.; Kwak, H.-B. Aging-induced Sarcopenia and Exercise. The Official Journal of the Korean Academy of Kinesiology 2017, 19, 43-59.
- Csapo, R.; Alegre, L.M. Effects of resistance training with moderate vs heavy loads on muscle mass and strength in the elderly: A meta-analysis. Scand J Med Sci Sports 2016, 26, 995-1006, doi:10.1111/sms.12536.
- Peterson, M.D.; Sen, A.; Gordon, P.M. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc 2011, 43, 249-258, doi:10.1249/MSS.0b013e3181eb6265.
- Trouwborst, I.; Verreijen, A.; Memelink, R.; Massanet, P.; Boirie, Y.; Weijs, P.; Tieland, M. Exercise and Nutrition Strategies to Counteract Sarcopenic Obesity. Nutrients 2018, 10, 605, doi:10.3390/nu10050605.
- Chen, H.T.; Chung, Y.C.; Chen, Y.J.; Ho, S.Y.; Wu, H.J. Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. J Am Geriatr Soc 2017, 65, 827-832, doi:10.1111/jgs.14722.
- Liao, C.D.; Tsauo, J.Y.; Lin, L.F.; Huang, S.W.; Ku, J.W.; Chou, L.C.; Liou, T.H. Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity: A CONSORT-compliant prospective randomized controlled trial. Medicine (Baltimore) 2017, 96, e7115, doi:10.1097/MD.0000000000007115.
- Gadelha, A.B.; Paiva, F.M.; Gauche, R.; de Oliveira, R.J.; Lima, R.M. Effects of resistance training on sarcopenic obesity index in older women: A randomized controlled trial. Arch Gerontol Geriatr 2016, 65, 168-173, doi:10.1016/j.archger.2016.03.017.
- Forbes, S.C.; Little, J.P.; Candow, D.G. Exercise and nutritional interventions for improving aging muscle health. Endocrine 2012, 42, 29-38, doi:10.1007/s12020-012-9676-1.
- Erlich, A.T.; Tryon, L.D.; Crilly, M.J.; Memme, J.M.; Moosavi, Z.S.M.; Oliveira, A.N.; Beyfuss, K.; Hood, D.A. Function of specialized regulatory proteins and signaling pathways in exercise-induced muscle mitochondrial biogenesis. Integr Med Res 2016, 5, 187-197, doi:10.1016/j.imr.2016.05.003.
- Short, K.R.; Vittone, J.L.; Bigelow, M.L.; Proctor, D.N.; Nair, K.S. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab 2004, 286, E92-101, doi:10.1152/ajpendo.00366.2003.
- Ko, I.G.; Jeong, J.W.; Kim, Y.H.; Jee, Y.S.; Kim, S.E.; Kim, S.H.; Jin, J.J.; Kim, C.J.; Chung, K.J. Aerobic exercise affects myostatin expression in aged rat skeletal muscles: a possibility of antiaging effects of aerobic exercise related with pelvic floor muscle and urethral rhabdosphincter. Int Neurourol J 2014, 18, 77-85, doi:10.5213/inj.2014.18.2.77.
- Bouaziz, W.; Schmitt, E.; Kaltenbach, G.; Geny, B.; Vogel, T. Health benefits of endurance training alone or combined with diet for obese patients over 60: a review. Int J Clin Pract 2015, 69, 1032-1049, doi:10.1111/ijcp.12648.
- Liberman, K.; Forti, L.N.; Beyer, I.; Bautmans, I. The effects of exercise on muscle strength, body composition, physical functioning and the inflammatory profile of older adults: a systematic review. Curr Opin Clin Nutr Metab Care 2017, 20, 30-53, doi:10.1097/MCO.0000000000000335.
- Porcari, J.P.; McLean, K.P.; Foster, C.; Kernozek, T.; Crenshaw, B.; Swenson, C. Effects of electrical muscle stimulation on body composition, muscle strength, and physical appearance. J Strength Cond Res 2002, 16, 165-172.
- Musumeci, G. The use of vibration as physical exercise and therapy. Journal of Functional Morphology and Kinesiology 2017, 2, 17.
- Scott, B.R.; Loenneke, J.P.; Slattery, K.M.; Dascombe, B.J. Exercise with blood flow restriction: an updated evidence-based approach for enhanced muscular development. Sports Med 2015, 45, 313-325, doi:10.1007/s40279-014-0288-1.
- Hughes, L.; Paton, B.; Rosenblatt, B.; Gissane, C.; Patterson, S.D. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med 2017, 51, 1003-1011, doi:10.1136/bjsports-2016-097071.
- Kokkinos, P. Physical activity, health benefits, and mortality risk. ISRN Cardiol 2012, 2012, 718789, doi:10.5402/2012/718789.
- Febbraio, M.A. Exercise metabolism in 2016: Health benefits of exercise - more than meets the eye! Nat Rev Endocrinol 2017, 13, 72-74, doi:10.1038/nrendo.2016.218.
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012, 8, 457-465, doi:10.1038/nrendo.2012.49.
- Febbraio, M.A.; Pedersen, B.K. Who would have thought—myokines two decades on. Nature Reviews Endocrinology 2020, 1-2.
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr Rev 2020, 41, bnaa016, doi:10.1210/endrev/bnaa016.
- Guo, A.; Li, K.; Xiao, Q. Sarcopenic obesity: Myokines as potential diagnostic biomarkers and therapeutic targets? Experimental Gerontology 2020, 111022.
- Kwon, J.H.; Moon, K.M.; Min, K.-W. Exercise-Induced Myokines can Explain the Importance of Physical Activity in the Elderly: An Overview. Healthcare 2020, 8, 378.
- Li, H.; Chen, Q.; Li, C.; Zhong, R.; Zhao, Y.; Zhang, Q.; Tong, W.; Zhu, D.; Zhang, Y. Muscle‐secreted granulocyte colony‐stimulating factor functions as metabolic niche factor ameliorating loss of muscle stem cells in aged mice. The EMBO journal 2019, 38, e102154.
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardi, M.; Munoz-Canoves, P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 2008, 7, 33-44, doi:10.1016/j.cmet.2007.11.011.
