Lactic acid fermentation of fresh fruit juices is a low-cost and sustainable process, that aims to preserve and even enhance the organoleptic and nutritional features of the raw matrices and extend their shelf life. Selected Lactic Acid Bacteria (LAB) were evaluated in the fermentation of various fruit juices, leading in some cases to fruit beverages, with enhanced nutritional and sensorial characteristics. Among LAB, Lactiplantibacillus (Lpb.) plantarum subsp. plantarum strains are quite interesting, regarding their application in the fermentation of a broad range of plant-derived substrates, such as vegetables and fruit juices, since they have genome plasticity and high versatility and flexibility. L. plantarum exhibits a remarkable portfolio of enzymes that make it very important and multi-functional in fruit juice fermentations. Therefore, L. plantarum has the potential for the production of various bioactive compounds, which enhance the nutritional value and the shelf life of the final product. In addition, L. plantarum can positively modify the flavor of fruit juices, leading to higher content of desirable volatile compounds.
- L. plantarum
- probiotics
- fruit juices
- health benefits
- enzymes
- phenolics
1. Introduction
2. Lactic Acid Fermentation of Fruit Juices
| Fruit Juices | Strains | Main Positive Effects | References |
|---|---|---|---|
| Mulberry juice (Morus nigra) |
L. plantarum, L. acidophilus, L. paracasei |
Increase in total anthocyanin, phenolic, antioxidant activity. | [22] |
| Pomegranate juice (Punica granatum L.) |
L. plantarum | Increase in antimicrobial activity. Volatile free fatty acids content increased. Better organoleptic properties and composition of volatile compounds. | [23] |
| Pomegranate juice (Punica granatum L.) |
L. plantarum | Improved sensorial characteristics. improved TPC and antioxidant activity. | [24] |
| Pomegranate juice (Punica granatum L.) |
L. paracasei | Improved sensorial characteristics. Improved TPC and antioxidant activity. | [13,25][13][25] |
| Cornelian cherry (Cornus mas L.) |
L. paracasei | Improved TPC and antioxidant activity. | [26] |
| Cashew apple juice (Anacardium occidentale L.) |
B. bifdum, B. longum subsp. infantis, L. plantarum, L. acidophilus, L. mesenteroides, L. johnsonii |
Improved antioxidant activity. Modification of the type and content of phenolic. Possible prebiotic action of phenolics to lactic acid bacteria. Contained prebiotic oligosaccharides mesenteroides) enhanced the growth of L. johnsonii. |
[27] |
| Cantaloupe melon (Cucumis melo L.) and cashew apple juice (Anacardium occidentale L.) |
L. casei | Emergence of new volatile compounds. | [28] |
| Apple juice | L. acidophilus, L. rhamnosus, L. casei, L. plantarum |
Generation of new aromatic compounds. | [29] |
| Apple juice | L. plantarum | Enhanced antioxidant capacity and bioavailability of polyphenols. | [30] |
| Elderberry juice (Sambucus nigra L.) |
L. plantarum | Improved composition of volatile compounds. | [20] |
| Jujube juice (Ziziphus jujuba Mill.) |
L. plantarum | Enhanced sensorial features. | [31] |
| Noni juice (Morinda citrifolia) |
L. casei, L. plantarum, B. longum, L. acidophilus | Slight increase in total antioxidant activity. ACE inhibitory potential decreased during fermentation. The content of ascorbic acid and antioxidant activity remained stable. | [32] |
| Cranberry juice (Vaccinium macrocarpon) |
L. paracasei | Synergistic and additive antibacterial effects of the combination of fermented cranberry juice and antibiotics. | [33] |
| Phyllanthus emblica fruit juice |
L. paracasei | Increased Total polyphenol content and antioxidant activity. | [34] |
| Jujube juice (Ziziphus jujuba Mill.) |
L. rhamnosus, L. plantarum, L. paracasei, L. mesenteroides, L. plantarum |
Increased flavonoid content. | [35] |
| Cactus pear juice (Opuntia fcus-indica) |
L. plantarum | Ameliorated insulin resistance. | [36] |
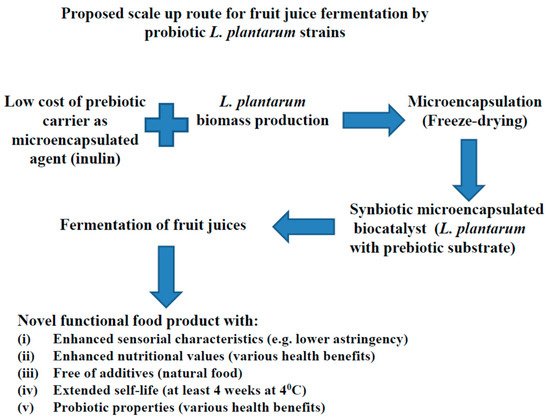
3. Main Advantages of L. plantarum Application in Food Fermentations
Application of L. plantarum Strains in Various Fruit Juices Fermentations
| Food Matrix | Commercial Name | Origin | Active Probiotic Culture |
|---|---|---|---|
| Fruit drink | Probi-Bravo Friscus | Sweden | L. plantarum, L. paracasei |
| Fruit drink | Danone-ProViva | Sweden, Finland | L. plantarum |
| Raw organic fruits and vegetable blend | Garden of Life RAW Organic Kids Probiotic | Florida, USA | L. gasseri, L. plantarum, L. casei, L. acidophilus |
| Fruit juice drink | GoodBelly | Colorado, USA | Lb. plantarum 299v |
| Fruit- and vegetable-based | KeVita active probiotic drink | Oxnard, USA | B. coagulans, L. rhamnosus, L. plantarum |
| Fruit juice | Healthy Life Probiotic juice | Australia | L. plantarum, L. casei |
References
- Oliveira, A.; Amaro, A.L.; Pintado, M. Impact of Food Matrix Components on Nutritional and Functional Properties of Fruit-Based Products. Curr. Opin. Food Sci. 2018, 22, 153–159.
- Mantzourani, I.; Plessas, S.; Odatzidou, M.; Alexopoulos, A.; Galanis, A.; Bezirtzoglou, E.; Bekatorou, A. Effect of a Novel Lactobacillus Paracasei Starter on Sourdough Bread Quality. Food Chem. 2019, 271, 259–265.
- Wong, W.-Y.; Chan, B.D.; Leung, T.-W.; Chen, M.; Tai, W.C.-S. Beneficial and Anti-Inflammatory Effects of Formulated Prebiotics, Probiotics, and Synbiotics in Normal and Acute Colitis Mice. J. Funct. Foods 2022, 88, 104871.
- James, A.; Wang, Y. Characterization, Health Benefits and Applications of Fruits and Vegetable Probiotics. CyTA-J. Food 2019, 17, 770–780.
- Vitali, B.; Minervini, G.; Rizzello, C.G.; Spisni, E.; Maccaferri, S.; Brigidi, P.; Gobbetti, M.; Di Cagno, R. Novel Probiotic Candidates for Humans Isolated from Raw Fruits and Vegetables. Food Microbiol. 2012, 31, 116–125.
- Kandylis, P.; Pissaridi, K.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A. Dairy and Non-Dairy Probiotic Beverages. Curr. Opin. Food Sci. 2016, 7, 58–63.
- Ranadheera, C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67.
- Ephrem, E.; Najjar, A.; Charcosset, C.; Greige-Gerges, H. Encapsulation of Natural Active Compounds, Enzymes, and Probiotics for Fruit Juice Fortification, Preservation, and Processing: An Overview. J. Funct. Foods 2018, 48, 65–84.
- Horáčková, Š.; Rokytová, K.; Bialasová, K.; Klojdová, I.; Sluková, M. Fruit Juices with Probiotics–New Type of Functional Foods. Czech J. Food Sci. 2018, 36, 284–288.
- Perricone, M.; Bevilacqua, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Challenges for the Production of Probiotic Fruit Juices. Beverages 2015, 1, 95–103.
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.A.; et al. Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858.
- Pontonio, E.; Montemurro, M.; Pinto, D.; Marzani, B.; Trani, A.; Ferrara, G.; Mazzeo, A.; Gobbetti, M.; Rizzello, C.G. Lactic Acid Fermentation of Pomegranate Juice as a Tool to Improve Antioxidant Activity. Front. Microbiol. 2019, 10.
- Plessas, S.; Nouska, C.; Karapetsas, A.; Kazakos, S.; Alexopoulos, A.; Mantzourani, I.; Chondrou, P.; Fournomiti, M.; Galanis, A.; Bezirtzoglou, E. Isolation, Characterization and Evaluation of the Probiotic Potential of a Novel Lactobacillus Strain Isolated from Feta-Type Cheese. Food Chem. 2017, 226, 102–108.
- Snyder, A.B.; Worobo, R.W. The Incidence and Impact of Microbial Spoilage in the Production of Fruit and Vegetable Juices as Reported by Juice Manufacturers. Food Control 2018, 85, 144–150.
- Rojo, M.C.; López, F.A.; Lerena, M.C.; Mercado, L.; Torres, A.; Combina, M. Evaluation of Different Chemical Preservatives to Control Zygosaccharomyces Rouxii Growth in High Sugar Culture Media. Food Control 2015, 50, 349–355.
- Panitsa, A.; Petsi, T.; Kandylis, P.; Kanellaki, M.; Koutinas, A.A. Tubular Cellulose from Orange Juice By-Products as Carrier of Chemical Preservatives; Delivery Kinetics and Microbial Stability of Orange Juice. Foods 2021, 10, 1882.
- Silveira, A.C.; Aguayo, E.; Artés, F. Shelf-Life and Quality Attributes in Fresh-Cut Galia Melon Combined with Fruit Juices. LWT-Food Sci. Technol. 2013, 50, 343–348.
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent Developments in Novel Shelf Life Extension Technologies of Fresh-Cut Fruits and Vegetables. Trends Food Sci. Technol. 2017, 64, 23–38.
- Filannino, P.; Tlais, A.Z.; Morozova, K.; Cavoski, I.; Scampicchio, M.; Gobbetti, M.; Di Cagno, R. Lactic Acid Fermentation Enriches the Profile of Biogenic Fatty Acid Derivatives of Avocado Fruit (Persea Americana Mill.). Food Chem. 2020, 317, 126384.
- Ricci, A.; Cirlini, M.; Levante, A.; Dall’Asta, C.; Galaverna, G.; Lazzi, C. Volatile Profile of Elderberry Juice: Effect of Lactic Acid Fermentation Using L. Plantarum, L. Rhamnosus and L. Casei Strains. Food Res. Int. 2018, 105, 412–422.
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Lactic Acid Fermentation Drives the Optimal Volatile Flavor-Aroma Profile of Pomegranate Juice. Int. J. Food Microbiol. 2017, 248, 56–62.
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of Lactobacillus Strains on Phenolic Profile, Color Attributes and Antioxidant Activities of Lactic-Acid-Fermented Mulberry Juice. Food Chem. 2018, 250, 148–154.
- Valero-Cases, E.; Nuncio-Jáuregui, N.; Frutos, M.J. Influence of Fermentation with Different Lactic Acid Bacteria and in Vitro Digestion on the Biotransformation of Phenolic Compounds in Fermented Pomegranate Juices. J. Agric. Food Chem. 2017, 65, 6488–6496.
- Mantzourani, I.; Kazakos, S.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Bekatorou, A.; Plessas, S. Potential of the Probiotic Lactobacillus Plantarum ATCC 14917 Strain to Produce Functional Fermented Pomegranate Juice. Foods 2019, 8, 4.
- Mantzourani, I.; Terpou, A.; Bekatorou, A.; Mallouchos, A.; Alexopoulos, A.; Kimbaris, A.; Bezirtzoglou, E.; Koutinas, A.A.; Plessas, S. Functional Pomegranate Beverage Production by Fermentation with a Novel Synbiotic L. Paracasei Biocatalyst. Food Chem. 2020, 308, 125658.
- Mantzourani, I.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Bekatorou, A.; Plessas, S. Production of a Potentially Synbiotic Fermented Cornelian Cherry (Cornus Mas L.) Beverage Using Lactobacillus Paracasei K5 Immobilized on Wheat Bran. Biocatal. Agric. Biotechnol. 2019, 17, 347–351.
- Vergara, C.M.d.A.C.; Honorato, T.L.; Maia, G.A.; Rodrigues, S. Prebiotic Effect of Fermented Cashew Apple (Anacardium Occidentale L) Juice. LWT-Food Sci. Technol. 2010, 43, 141–145.
- de Godoy Alves Filho, E.; Rodrigues, T.H.S.; Fernandes, F.A.N.; Pereira, A.L.F.; Narain, N.; de Brito, E.S.; Rodrigues, S. Chemometric Evaluation of the Volatile Profile of Probiotic Melon and Probiotic Cashew Juice. Food Res. Int. 2017, 99, 461–468.
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 Lactic Acid Bacteria on the Flavor Profile of Fermented Apple Juice. Food Biosci. 2019, 27, 30–36.
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced Antioxidant Activity for Apple Juice Fermented with Lactobacillus Plantarum ATCC14917. Molecules 2019, 24, 51.
- Li, T.; Jiang, T.; Liu, N.; Wu, C.; Xu, H.; Lei, H. Biotransformation of Phenolic Profiles and Improvement of Antioxidant Capacities in Jujube Juice by Select Lactic Acid Bacteria. Food Chem. 2021, 339, 127859.
- Nayak, B.S.; Marshall, J.R.; Isitor, G.; Adogwa, A. Hypoglycemic and Hepatoprotective Activity of Fermented Fruit Juice of Morinda Citrifolia (Noni) in Diabetic Rats. Evid. -Based Complementary Altern. Med. 2010, 2011.
- Mantzourani, I.; Bontsidis, C.A.; Plessas, S.; Alexopoulos, A.; Theodoridou, E.; Tsigalou, C.; Voidarou, C.; Douganiotis, G.; Kazakos, S.L.; Stavropoulou, E. Comparative Susceptibility Study against Pathogens Using Fermented Cranberry Juice and Antibiotics. Front. Microbiol. 2019, 10, 1294.
- Peerajan, S.; Chaiyasut, C.; Sirilun, S.; Chaiyasut, K.; Kesika, P.; Sivamaruthi, B.S. Enrichment of Nutritional Value of Phyllanthus Emblica Fruit Juice Using the Probiotic Bacterium, Lactobacillus Paracasei HII01 Mediated Fermentation. Food Sci. Technol. 2016, 36, 116–123.
- Zhao, M.-N.; Zhang, F.; Zhang, L.; Liu, B.-J.; Meng, X.-H. Mixed Fermentation of Jujube Juice (Ziziphus Jujuba Mill.) with L. Rhamnosus GG and L. Plantarum-1: Effects on the Quality and Stability. Int. J. Food Sci. Technol. 2019, 54, 2624–2631.
- Verón, H.E.; Cano, P.G.; Fabersani, E.; Sanz, Y.; Isla, M.I.; Espinar, M.T.F.; Ponce, J.V.G.; Torres, S. Cactus Pear (Opuntia Ficus-Indica) Juice Fermented with Autochthonous Lactobacillus Plantarum S-811. Food Funct. 2019, 10, 1085–1097.
- Shubhada, N.; Rudresh, D.L.; Jagadeesh, S.L.; Prakash, D.P.; Raghavendra, S. Fermentation of Pomegranate Juice by Lactic Acid Bacteria. Int J Curr Microbiol Appl Sci 2018, 7, 4160–4173.
- Muhialdin, B.J.; Kadum, H.; Hussin, A.S.M. Metabolomics Profiling of Fermented Cantaloupe Juice and the Potential Application to Extend the Shelf Life of Fresh Cantaloupe Juice for Six Months at 8 C. Food Control 2021, 120, 107555.
- Srisukchayakul, P.; Charalampopoulos, D.; Karatzas, K.A. Study on the Effect of Citric Acid Adaptation toward the Subsequent Survival of Lactobacillus Plantarum NCIMB 8826 in Low PH Fruit Juices during Refrigerated Storage. Food Res. Int. 2018, 111, 198–204.
- Sheehan, V.M.; Ross, P.; Fitzgerald, G.F. Assessing the Acid Tolerance and the Technological Robustness of Probiotic Cultures for Fortification in Fruit Juices. Innov. Food Sci. Emerg. Technol. 2007, 8, 279–284.
- Vinderola, C.G.; Bailo, N.; Reinheimer, J.A. Survival of Probiotic Microflora in Argentinian Yoghurts during Refrigerated Storage. Food Res. Int. 2000, 33, 97–102.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514.
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; De Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890.
- Bucka-Kolendo, J.; Soko\lowska, B. Lactic Acid Bacteria Stress Response to Preservation Processes in the Beverage and Juice Industry. Acta Biochim. Pol. 2017, 64, 459–464.
- Gaucher, F.; Bonnassie, S.; Rabah, H.; Marchand, P.; Blanc, P.; Jeantet, R.; Jan, G. Adaptation of Beneficial Propionibacteria, Lactobacilli, and Bifidobacteria Improves Tolerance toward Technological and Digestive Stresses. Front. Microbiol. 2019, 10, 841.
- Mitropoulou, G.; Nedovic, V.; Goyal, A.; Kourkoutas, Y. Immobilization Technologies in Probiotic Food Production. J. Nutr. Metab. 2013, 2013.
- Colín-Cruz, M.A.; Pimentel-González, D.J.; Carrillo-Navas, H.; Alvarez-Ramírez, J.; Guadarrama-Lezama, A.Y. Co-Encapsulation of Bioactive Compounds from Blackberry Juice and Probiotic Bacteria in Biopolymeric Matrices. LWT 2019, 110, 94–101.
- Dimitrellou, D.; Kandylis, P.; Lević, S.; Petrović, T.; Ivanović, S.; Nedović, V.; Kourkoutas, Y. Encapsulation of Lactobacillus Casei ATCC 393 in Alginate Capsules for Probiotic Fermented Milk Production. LWT 2019, 116, 108501.
- Sarao, L.K.; Arora, M. Probiotics, Prebiotics, and Microencapsulation: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 344–371.
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of Probiotics for Gastrointestinal Delivery. J. Control. Release 2012, 162, 56–67.
- Costa, M.G.M.; Fonteles, T.V.; de Jesus, A.L.T.; Rodrigues, S. Sonicated Pineapple Juice as Substrate for L. Casei Cultivation for Probiotic Beverage Development: Process Optimisation and Product Stability. Food Chem. 2013, 139, 261–266.
- Racioppo, A.; Corbo, M.R.; Piccoli, C.; Sinigaglia, M.; Speranza, B.; Bevilacqua, A. Ultrasound Attenuation of Lactobacilli and Bifidobacteria: Effect on Some Technological and Probiotic Properties. Int. J. Food Microbiol. 2017, 243, 78–83.
- Bevilacqua, A.; Casanova, F.P.; Petruzzi, L.; Sinigaglia, M.; Corbo, M.R. Using Physical Approaches for the Attenuation of Lactic Acid Bacteria in an Organic Rice Beverage. Food Microbiol. 2016, 53, 1–8.
- Gawkowski, D.; Chikindas, M.L. Non-Dairy Probiotic Beverages: The next Step into Human Health. Benef. Microbes 2013, 4, 127–142.
- Arellano, K.; Vazquez, J.; Park, H.; Lim, J.; Ji, Y.; Kang, H.-J.; Cho, D.; Jeong, H.W.; Holzapfel, W.H. Safety Evaluation and Whole-Genome Annotation of Lactobacillus Plantarum Strains from Different Sources with Special Focus on Isolates from Green Tea. Probiotics Antimicrob. Proteins 2020, 12, 1057–1070.
- Szutowska, J. Functional Properties of Lactic Acid Bacteria in Fermented Fruit and Vegetable Juices: A Systematic Literature Review. Eur. Food Res. Technol. 2020, 246, 357–372.
- Wang, Q.; Sun, Q.; Wang, J.; Qiu, X.; Qi, R.; Huang, J. Lactobacillus Plantarum 299v Changes MiRNA Expression in the Intestines of Piglets and Leads to Downregulation of LITAF by Regulating Ssc-MiR-450a. Probiotics Antimicrob. Proteins 2021, 1–13.
- Oh, Y.J.; Kim, T.S.; Moon, H.W.; Lee, S.Y.; Lee, S.Y.; Ji, G.E.; Hwang, K.T. Lactobacillus Plantarum PMO 08 as a Probiotic Starter Culture for Plant-Based Fermented Beverages. Molecules 2020, 25, 5056.
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus Plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. Int. 2018, 2018.
- De Vries, M.C.; Vaughan, E.E.; Kleerebezem, M.; de Vos, W.M. Lactobacillus Plantarum—Survival, Functional and Potential Probiotic Properties in the Human Intestinal Tract. Int. Dairy J. 2006, 16, 1018–1028.
- Wang, S.-Y.; Zhu, H.-Z.; Lan, Y.-B.; Liu, R.-J.; Liu, Y.-R.; Zhang, B.-L.; Zhu, B.-Q. Modifications of Phenolic Compounds, Biogenic Amines, and Volatile Compounds in Cabernet Gernishct Wine through Malolactic Fermentation by Lactobacillus Plantarum and Oenococcus Oeni. Fermentation 2020, 6, 15.
- Lanza, B.; Zago, M.; Di Marco, S.; Di Loreto, G.; Cellini, M.; Tidona, F.; Bonvini, B.; Bacceli, M.; Simone, N. Single and Multiple Inoculum of Lactiplantibacillus Plantarum Strains in Table Olive Lab-Scale Fermentations. Fermentation 2020, 6, 126.
- Campaniello, D.; Speranza, B.; Bevilacqua, A.; Altieri, C.; Rosaria Corbo, M.; Sinigaglia, M. Industrial Validation of a Promising Functional Strain of Lactobacillus Plantarum to Improve the Quality of Italian Sausages. Microorganisms 2020, 8, 116.
- Alemneh, S.T.; Emire, S.A.; Hitzmann, B. Teff-Based Probiotic Functional Beverage Fermented with Lactobacillus Rhamnosus and Lactobacillus Plantarum. Foods 2021, 10, 2333.
- Ibrahim, F.; Ouwehand, A.C. The Genus Lactobacillus. Lact. Acid Bact. Microbiol. Funct. Asp. 2019, 47.
- Prete, R.; Long, S.L.; Joyce, S.A.; Corsetti, A. Genotypic and Phenotypic Characterization of Food-Associated Lactobacillus Plantarum Isolates for Potential Probiotic Activities. FEMS Microbiol. Lett. 2020, 367, fnaa076.
- Shahidi, F.; Peng, H. Bioaccessibility and Bioavailability of Phenolic Compounds. J. Food Bioact. 2018, 4, 11–68.
- Boekhorst, J.; Wels, M.; Kleerebezem, M.; Siezen, R.J. The Predicted Secretome of Lactobacillus Plantarum WCFS1 Sheds Light on Interactions with Its Environment. Microbiology 2006, 152, 3175–3183.
- Park, J.-B.; Lim, S.-H.; Sim, H.-S.; Park, J.-H.; Kwon, H.-J.; Nam, H.S.; Kim, M.-D.; Baek, H.-H.; Ha, S.-J. Changes in Antioxidant Activities and Volatile Compounds of Mixed Berry Juice through Fermentation by Lactic Acid Bacteria. Food Sci. Biotechnol. 2017, 26, 441–446.
- Huang, R.; Xu, C. An Overview of the Perception and Mitigation of Astringency Associated with Phenolic Compounds. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1036–1074.
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus Plantarum and Its Probiotic and Food Potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122.
- Al-Tawaha, R.; Meng, C. Potential Benefits of Lactobacillus Plantarum as Probiotic and Its Advantages in Human Health and Industrial Applications: A Review. Adv. Environ. Biol 2018, 12, 16–27.
- Li, P.; Li, X.; Gu, Q.; Lou, X.; Zhang, X.; Song, D.; Zhang, C. Comparative Genomic Analysis of Lactobacillus Plantarum ZJ316 Reveals Its Genetic Adaptation and Potential Probiotic Profiles. J. Zhejiang Univ. -Sci. B 2016, 17, 569–579.
- Moradi, M.; Molaei, R.; Guimarães, J.T. A Review on Preparation and Chemical Analysis of Postbiotics from Lactic Acid Bacteria. Enzym. Microb. Technol. 2021, 143, 109722.
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370.
- Putnik, P.; Pavlić, B.; Šojić, B.; Zavadlav, S.; Žuntar, I.; Kao, L.; Kitonić, D.; Kovačević, D.B. Innovative Hurdle Technologies for the Preservation of Functional Fruit Juices. Foods 2020, 9, 699.
- Tanganurat, P. Probiotics Encapsulated Fruit Juice Bubbles as Functional Food Product. Cell 2020, 4, 5.
- Zhu, W.; Lyu, F.; Naumovski, N.; Ajlouni, S.; Ranadheera, C.S. Functional Efficacy of Probiotic Lactobacillus Sanfranciscensis in Apple, Orange and Tomato Juices with Special Reference to Storage Stability and In Vitro Gastrointestinal Survival. Beverages 2020, 6, 13.
- Todorov, S.D.; Franco, B.D.G.D.M. Lactobacillus Plantarum: Characterization of the Species and Application in Food Production. Food Rev. Int. 2010, 26, 205–229.
- Filannino, P.; Cardinali, G.; Rizzello, C.G.; Buchin, S.; De Angelis, M.; Gobbetti, M.; Di Cagno, R. Metabolic Responses of Lactobacillus Plantarum Strains during Fermentation and Storage of Vegetable and Fruit Juices. Appl. Environ. Microbiol. 2014, 80, 2206–2215.
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J. Agric. Food Chem. 2000, 48, 4581–4589.
- Landete, J.M.; Rodriguez, H.; De Las Rivas, B.; Munoz, R. High-Added-Value Antioxidants Obtained from the Degradation of Wine Phenolics by Lactobacillus Plantarum. J. Food Prot. 2007, 70, 2670–2675.
- Mantzourani, I.; Nouska, C.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Panayiotidis, M.I.; Galanis, A.; Plessas, S. Production of a Novel Functional Fruit Beverage Consisting of Cornelian Cherry Juice and Probiotic Bacteria. Antioxidants 2018, 7, 163.
- Hashemi, S.M.B.; Khaneghah, A.M.; Barba, F.J.; Nemati, Z.; Shokofti, S.S.; Alizadeh, F. Fermented Sweet Lemon Juice (Citrus Limetta) Using Lactobacillus Plantarum LS5: Chemical Composition, Antioxidant and Antibacterial Activities. J. Funct. Foods 2017, 38, 409–414.
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of Blueberry and Blackberry Juices Using Lactobacillus Plantarum, Streptococcus Thermophilus and Bifidobacterium Bifidum: Growth of Probiotics, Metabolism of Phenolics, Antioxidant Capacity in Vitro and Sensory Evaluation. Food Chem. 2021, 348, 129083.
- Dey, G. Non-Dairy Probiotic Foods: Innovations and Market Trends. In Innovations in Technologies for Fermented Food and Beverage Industries; Springer: Berlin/Heidelberg, Germany, 2018; pp. 159–173.
