NK cells are usually defined as immune cells that belong to the innate immune response. They were described as cells capable of killing several tumour cell lines without previous antigen presentation. It was shown then that NK cells lack the TCR and BCR receptors capable of binding specific antigens, and consequently, it was assumed that these cells lack antigen recognition.
- NK cells
- memory NK cells
- antiviral response
- killing inhibitory receptors
- killing receptors
- antibody-dependent cell cytotoxicity (ADCC)
1. Different Receptors Involved in NK Cell Activation and Inhibition involved in Pathogen Elimination
| Species | NK Cell Receptor | Natural Cellular Ligand | Effect on NK Function |
|---|---|---|---|
| Mouse | NCR46 | Vimentin, viral antigen | Activating |
| GAG | |||
| NKG2D | RAE 1a, b, d, g | ||
| H60a-c, MULT1 | |||
| DNAM1 | CD112, CD155 | ||
| CD94/NKG2C | Qa-1 | ||
| CD94/NKG2E | Qa-1 | ||
| CD16 | IgG | ||
| LY49D | H2Dd | ||
| LY49H | CMV glycoprotein | ||
| Ly49P | CMV glycoprotein | ||
| CD94/NKG2A | Qa-1 | Inhibiting | |
| KLRG1 | Cadherins (E, N, and R) | ||
| LY49A | H2Dd, H2Dk | ||
| LY49I | H2Dk | ||
| NK1.1 | Lectin | ||
| CD244 | CD48 | Activating/Inhibiting | |
| Human | CD94/NKG2C CD94/NKG2D | DAP-12/HLA | Activating |
| MICA A/B, ULPB1-6 | |||
| NCR30 | B7-H6, BAT 3, GAG | ||
| NCR44 | Heparan sulfate, heparin. GAG | ||
| NCR46 | Vimentin, Viral antigens, GAG | ||
| IgG | |||
| CD16 | HLA-C2 | ||
| KIR2DS1 | |||
| KIR2DS2 | HLA-C | ||
| KIR2DS4 | HLA-C | ||
| KIR2DS5 | HLA-C | ||
| KIR3DS1 | HLA-B, HLA-F | ||
| KIR2DL4 | HLA-G | ||
| DNAM1 | CD112, CD155 | ||
| NTBA | NTB-A, viral antigens | ||
| CD94/NKG2A | HLA-E | Inhibiting | |
| KIR3DL1/2 | HLA-C | ||
| KIR2DL2/3 | HLA-B/HLA-C | ||
| KIR2DL1 | HLA-C | ||
| KLRG1 | Cadherins (E, N, and R) | ||
| ILT2 | HLA-E | ||
| CD244 | CD48 | Activating/Inhibiting |
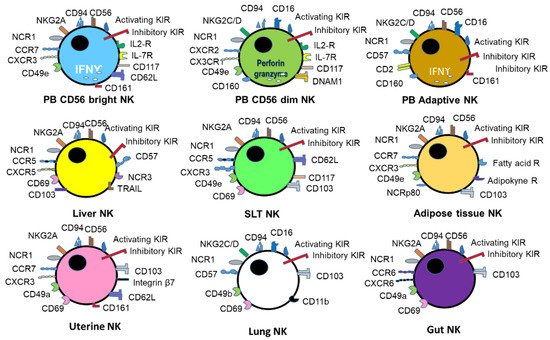
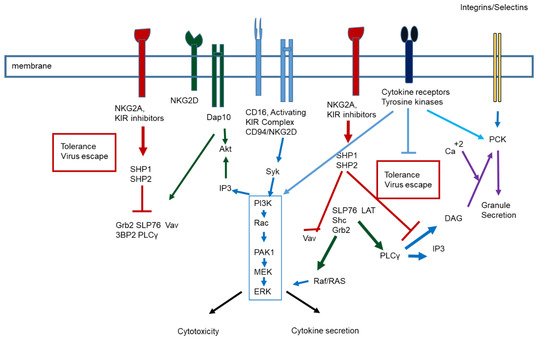
2. Virus infection and immune response
Innate and adaptive immune responses are essential to overcome SARS CoV2 infection [1][2][67-68]. An impaired innate immune response leads to an exacerbated cytokine inflammatory response (cytokine storm) that induce organ dysfunction and cell death, jeopardising the host response against the virus [1][2][67-68]. The hypoxia caused by the viral infection induces hypoxia-inducing factor (HIF), magnifying the inflammatory response [1][3][9, 67]. NK cells may contribute to the cytokine storm generated by a viral infection and may also aid in decreasing the effective adaptative response [3][9].
One of the reported events in COVID-19 infection is the decrease in circulating lymphocyte populations and several viral diseases [1][2][67-68]. The reduction in lymphocyte number in the peripheral blood is due to the increased traffic to lung tissue [1][2][67-68]. The number of NK cells decreases in peripheral blood, and the number of cells increases in adaptive-like tissue residue in the lung [1][2][67-68]. NK cells seem essential in the first stages of virus infection to the lungs since NK cells migrate along with macrophages and neutrophils into the lung due to the secretion of chemokines and IL-6 [1][2][67-68]. The production of IFNγ by NK cells is crucial to decrease viral load; however, the activation of the cells by FcR binding of IgG1 and IgG3, secreted by B cells, of NK cells and neutrophils induces more cell death and complement consumption [1][2][67-68]. Moreover, neutrophil FcR activation causes NETosis increasing inflammation and tissue damage [1][2][4][67-69]. Thus, danger signals are crucial in lung immunopathology.
3. NK cells in SARS CoV-2 infection
Several reports have been on NK cell numbers and peripheral blood subpopulations in SARS CoV-2 infection. A recent review discussed that non-conventional T cell responses were not thoroughly analysed in other coronavirus infections, including SARS CoV-1[1] [67]. The role of NKT and Tϒδ subpopulations in memory responses was partially studied [1][67]. Animal models may aid in assessing markers and cell populations; however, the mouse model is partly helpful due to the lack of expression of ACE2 receptor.
NKG2D expression and high perforin and granzyme B levels are observed in CD56 bright NK cells with COVID severity as lowered in SARS CoV2 severe patients [4][70]. This subpopulation, however, can be suppressed by IL-10 and TGF-β secreted by immune cells or tissue cells[5][6][7][8] [70-73]. Blocking TGF β could restore NK cell activity. T regulatory cells are involved in NK cell activation by decreasing NKG2D expression, which may affect the response of these cells in viral infections [5][6][7][8][70-73]. In addition, TGF β induces tolerogenic responses on NK cells. IFN is critical to the antiviral response against the virus, especially for NK cells[9] [74]. It would be interesting to assess if the production of type I IFN would decrease the inhibitory effect of TGFβ as proposed by Bastard and coworkers [10][75] with the increased incidence of anti-IFN antibodies in elderly individuals. The antibodies against IFN-α2 were also detected in convalescent plasma[11] [76].
Another interesting hypothesis has been discussed in which the glycoprotein content of the S protein and heparan sulfate binding in tissue could induce an increased expression of KIR inhibitory receptors [12][13][77-78]. Heparan sulfate and heparanase have been involved in NK cytotoxic responses[14][15] [79-80]. Heparanase activity, degrading membrane heparin sulfate is critical in cell activation, cytotoxicity, migration (probably due to CD44 receptor) and cytokine production[14] [79]. It can be suggested that Scoronavirus S protein binding to heparin sulfate may impair NK cell activation and consequently decrease the antiviral response.
In Ebola infection, it has been shown that the glycosylated Ebola glycoprotein can bind to homing receptors and in dendritic cells to DC-SIGN [16][81]. The interaction of these glycoproteins may affect cell tissue migration and NK cell response[14][15] [79-80] and is crucial for viral clearance [3][9][14][15][16][17][9, 17, 79-82].
Several reports in the literature suggest that CD8 cells cytotoxic responses against SARS CoV2 can protect against viral infection or severe disease [1][17][67, 82]. These CD8 responses may be related to alpha coronavirus infections or other pathogens [1][17][67, 82]. However, the researchers’ main question refers to MHC class I restriction; however, other non HLA class I mediated responses could be essential to generate memory response [17][18][82-83]. Moreover, one can envision that low viral load infection can develop an effective memory response, and high viral load infection may induce cytokine storm and, consequently, an impaired response.
If the individual is exposed to a high viral load, the probability of infecting target cells and the quick progression of the infection may induce an exacerbated immune response (cytokine storm) [1][67]. The excessive immune response could also be observed in individuals with no viral protective CD8, T and NK cell responses [1][2][4][5][6][7][8][9][10][11][12][13][14][15][16][18][67-83]. The marked production of cytokines may induce tolerogenic CD4 T cells to inhibit the effective antiviral immune response [19][84].
NK therapy clinical trials have recently been designed to treat SARS Co-2 infection[20][21][22][23] [85-88]. The therapy is based in vitro, stimulating NK cells obtained from 1) peripheral blood mononuclear cells, 2) NK cells generated from stem cell precursors, 3) genetically modified NK cell lines[20][21][22][23] [85-88]. Herrera et al. [23][88]. Recently described the presence of memory NK cells of convalescent donors after adoptive therapy. The first, FDA-approved cell therapy, is based on an allogeneic cryopreserved NK cell therapy. Cell immunotherapy is based on NK cells stimulation used for cancer immunotherapy. The cells are derived from human placental CD34+ cells and expanded and stimulated in vitro (CYNK-001). Safety and efficacy clinical with CYNK-001 are underway for patients with moderate SARS CoV-2 infection (NCT04365101). The other clinical trial involves chimaeric antigen receptor (CAR)-NK cell therapy on SARS CoV-2 infected patients at an early stage (up to 14 days symptoms). In vitro studies have shown the effectiveness of CAR NK cells are directed against the Spike protein presented by infected cell lines [24][89]. However, it would be naïve to think that NK cell therapy responses would be in the clinic due to the high cost of the therapy.
Another treatment refers to NK cells derived from the umbilical cord and is genetically modified to express NKG2D-ACE2 CARs (NCT04324996). These cells secrete an IL-15 antagonist and a soluble cFV that neutralises GM-CSF. GM-CSF was shown to be a crucial cytokine associated with the physiopathology of the disease, and it also modulates CD4+ Th1 cells. Another approach to actívate the cells is by managing checkpoints[25] [90]. Maybe specific antibodies against inhibitory receptors aside from PD1 and CTLA4 would be helpful. Biological checkpoint inhibitor treatment may increase NK cytotoxicity and decrease the probable tolerogenic response.
Antibodies against the SARS CoV-2 virus generate immune complexes[26] [91]. These immune complexes may aid viral immunopathogenesis since the complex enhance neutrophil activation and tissue damage [25][27][28][91-93]. It could be suggested that IgG immune complexes are involved in chronicity and severity since they can suppress effective NK cell responses, as shown in cancer therapy[29] [94]. However, the induction of adaptative responses through virus exposure of vaccine treatment could modify the response of NK cells [30][31][19, 52]. Even though hepatic manifestations of SARS CoV-2 are not very common in humans, there are descriptions of hepatitis following coronavirus infection in animals. The tissue-specific immune response differs in different models and may be challenging to ascertain the importance of NK cells.
During pregnancy, conventional NK cells protect pathogens and protect decidual NK cells and bystander cells [32][6]. The tissue, however, expresses ACE2 receptors, which are involved in SARS CoV-2 infection [32][1][6, 67, 95]. Therefore, uterine infection of the virus affects local NK cells and may hamper fetal growth and survival[32][33][34] [6, 95-96]. On the other hand, vaccines have protected pregnant women, and neonatal protective antibodies have been detected [35][97].
Exhaustion markers on NK cells, expression of PD-1 has been reported in SARS CoV2 patients [36][98]. The CD56 bright subpopulation decreases while the CD56 dim subpopulation increases. Varchetta and coworkers [2][68] showed that this ratio in SARS CoV2 infected patients that died. In the survivors, there is a decrease in the expression of CD69, TIM-3 and PD1 at convalescence [2][68]. There is a parallel decrease in secretory IL6, IL-8 and IL-1β [2][68]. One may envision that the memory responses of NK cells are affected in the patients that did not survive. However, this decrease in memory response could be due to another infection or comorbidities responsible for this impairment [37][38][39][40][99-102]. As an example, Couturier and Lewis[41] [63] were able to show that, in HIV infection, the involvement of adipose tissue and CD4 and NK and NKT cells is crucial for virus latency. One may envision that the penetrance of antiretroviral therapies in adipose tissue may predispose that this organ is a reservoir for the virus. In addition, essential changes in adipokines have been described upon antiretroviral treatment with the migration of adipose tissue. Migration could be a secondary event related to viral infection and persistence. CD8 response may be essential to understand the protective response to coronavirus in these patients.
Probably the metabolism of NK cells is also affected by the viral infection. In a murine model of Friend retrovirus, Littwitz-Salomon and coworkers [42][64] showed that iron was crucial for NK cell functions and affected the viral infection. Electrolyte impairment, potassium, chloride and sodium, have been described in SARS CoV-2 infection[1] [67]. Ferritin is a known predictor of SARS CoV-2 severity [43][44][103-104]. The deleterious effects of iron can probably be observed by the increased cell death due, at least in part, to ferroptosis[45] [105]. Iron deficiency could be partially involved in an impaired NK response in severe patients. IFN γ produced by NK cells can also induce NETosis, enhancing cell death [45][105]. However, abnormal potassium transport is related to hepatitis B virus-associated acute-on-chronic liver failure in mice with experimental fulminant hepatitis [46][106]. More research is required on this topic since potassium channels are essential in NK cytotoxic response since Kctd9-deficient mice exhibited an impaired NK maturation. These cells produce insufficient IFN-γ and granzyme B upon stimulation and, therefore, a decreased cytotoxic response against tumour or virus-infected cells[46] [106].
Tables 23 and 34 provide a general outlook of the involvement of NK cells, NK cells receptors in viral infections, and different mechanisms of NK cell memory induced not only by the viral infection itself but also by cytokines and vaccines. The induction of vaccines’ T, B and NK memory responses should be considered a priority since the protection may be efficient and long-lasting. The response of NK cells to SARS CoV-2 vaccines is still ongoing research dependent upon cellular immune assays[47] [107]. Most memory immune assays in SARS CoV-2 have validated CD4 memory responses and have been less effective in assessing CD8 and NK memory responses. However, several groups may provide the required tools to unravel the effective SARS CoV-2 immune response puzzle.
| Virus Infection-Induced Memory |
NK Cell Receptor | NK Peripheral | Tissue-Resident NK Cells |
|---|---|---|---|
| CMV | CD94/NKG2C+ CD57+, KIR2DS4, KIR2DS2, KIR3DS1. |
Increased peripheral NK cells in elderly individuals. Induction of CD57+ from CD56dim CD57-cells | Impairment of tissue to peripheral ILC cells |
| Dengue virus | CD94/NKG2C+. Inhibition of memory through KIR3DL1 by NS1 viral protein. | It increased peripheral CD56 bright cells. | Skin homing CLA+ NK cell phenotype |
| Ebola virus | CD94/NKG2C+ CD57+ | Increase in CD56 neg CD16pos supopulations | Active liver NK cells |
| HIV | CD94/NKG2C+ CD57+ | Increased frequencies of CD16pos CD56 ng NK cells | Lymph node, liver, placenta activated by infected cells |
| Hepatitis C | CD57+ KLRG1+ | Increased NCR46, CD56 bright | Active liver NK cells |
| Influenza virus | CD16+CD49a+CXCR3+ | Reduced CD56 bright | Increase of lung NK cells |
| SARS-CoV-2 | CD56di, NKG2C, Ksp37+ | Increase of CD56dim CD57+ cells | Active lung NK cells, Decidual, liver |
| Memory | NK Cell Receptor | Notes |
|---|---|---|
| Induced by virus | CD94/NKG2C+ CD57+ | HCMV induced memory. Present in young individuals, less probable on elders. Virus-induced mature NK cells undergo homeostatic cell division. They enhanced cytotoxic response and ADCC. |
| Infection | KIR2DS4, KIR2DS2, | |
| Human | KIR3DS1 | |
| Mouse | Ly49H+/DAP12 | NK cells that quickly respond to virus challenge. Other virus-binding receptors may be involved. |
| DNAM-1 | ||
| CXCR6+ | ||
| Cytokine-induced memory | IL-12R, IL-15R, IL-18R | Stimulation with IL-12, IL-15, IL-18 cytokines induces a pool of long-lived NK cells with enhanced cytokine reactivity and can kill virus cells. Increase ADCC. |
| Mouse and Human | ||
| Vaccine-induced | NKG2D + CXCR6+ | Influenza, BcG, Ebola, SARS-CoV-2 |
| Memory | ||
| Mouse and human |
The exciting issue to further investigate is NK memory cells and cell plasticity-based upon the epigenetic modulations [48][108]. The main problem to induce effective memory NK responses is ageing.
Recently, the probability of combined viral infection, primarily in the elder population, has prompted the sanitary authorities to vaccinate against influenza and coronavirus. However, Achdout and coworkers [49][46] pointed out that a combined protective response can be observed with influenza vaccine rather than SARS CoV-2 vaccine. It could be envisioned that a combined protein vaccine for SARS CoV-2 may be necessary to maintain memory responses for more extended periods in the risk populations.
