Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 1 by Takashi Tsuchiya.
Glutathione (GSH) is a tripeptide composed of glutamic acid, cysteine, and glycine. It is one of the strongest antioxidants in the body and important for adjusting immune function. Cystine and theanine (γ-glutamylethylamide) provide substrates of GSH, cysteine and glutamic acid, promoting the synthesis of GSH.
- cystine
- theanine
- glutathione (GSH)
- surgical stress
1. Amino Acids Cystine and Theanine
Cystine is a dimer of the sulfur-containing amino acid cysteine formed by disulfide bonding, and it is contained in many food materials including meat [21][1]. It is abundant in keratin, which is protein of the hair and nails [22][2]. Cystine incorporated into cells is reduced by thioredoxin (TRX) and becomes cysteine [23][3]. Theanine is an umami ingredient contained in tea [24][4]. Theanine is decomposed to glutamic acid and ethylamine in the body [25][5]. After entering cells, cysteine and glutamic acid are used together with glycine for the synthesis of GSH, one of substances with the strongest antioxidant effects in the body [26][6]. It has been reported that the addition of cystine to macrophages collected from human peripheral blood promotes intracellular GSH synthesis in a dose-dependent manner, and the addition of a theanine metabolite, glutamic acid, further increases GSH synthesis [27][7]. GSH also has important action for adjusting immune function, but a decrease in GSH under strong stress and inflammation was reported [28,29][8][9]. Accordingly, it is predicted that the promotion of GSH synthesis in a patient’s condition, reducing GSH, will strengthen the biological defense reaction.
2. Influence of Cystine/Theanine Administration on Exercise Load in Athletes
In athletes, the decline of immunity and poor physical condition occur after performing an exercise load stronger than normal, and the inhibitory effects of cystine/theanine have been reported. Murakami et al. set a practice menu applying a strong load of daily running distance longer than normal for 16 university long distance runners and divided the runners into a group ingesting 700 mg of cystine and 280 mg of theanine from 7 days before initiation of a training camp to 9 days after initiation (C/T group) and a group ingesting a placebo (control group) [30][10]. The degree of increase in the granulocyte count after the exercise load was significantly smaller in the C/T group on day 1 of camp, and the degree of decrease in the lymphocyte count was also significantly smaller in the C/T group. It was presumed that cystine/theanine inhibited the excessive inflammatory reaction and suppressed the decline of immunological function. In another report, Murakami et al. surveyed the effects of cystine/theanine on an exercise load stronger than normal in long-distance runners [31][11]. Fifteen runners were divided into a group ingesting 700 mg of cystine and 280 mg of theanine for 10 days before strong exercise (C/T group) and a group ingesting a placebo (control group). The rate of granulocytes significantly increased on day 10 of the camp in the control group, but no significant increase was noted in the C/T group. The rate of lymphocytes slightly decreased in the control group (p = 0.08), but no decrease was noted in the C/T group. CRP significantly increased in the control group, but no increase was noted in the C/T group. Based on this result, they also reported that cystine/theanine are effective for maintenance of the body condition by preventing strong exercise load-induced decline of immunity and excess inflammation. Similarly, Kawada et al. investigated the influence of cystine/theanine on immune function after a strong exercise load [32][12]. They divided 15 body builders into two groups. The C/T group orally ingested 700 mg of cystine and 280 mg of theanine and the control group orally ingested a placebo for 2 weeks. Both groups performed normal training in the first week (three times a week) and twofold training: six times/week in the second week and NK cell activity was measured before the initiation of training and on days 7 and 14. There was no change in NK cell activity between before training and day 7 in both groups, but when the frequency of exercise increased by two times, NK activity decreased to 69.2% of that before training in the control group, whereas it was 101.7% in the C/T group, demonstrating no decrease, being significantly different from that in the control group. This clarified that cystine/theanine restore NK cell activity reduced by the stress of performing exercise loads stronger than normal.
Based on the above reports, it was concluded that cystine/theanine administration promoting GSH synthesis exhibits stress-reducing effects after exercise loads stronger than normal.
3. Application of Cystine/Theanine to Patients Undergoing Surgery
Cystine/theanine, which inhibit excess inflammation after strong exercise loads and prevent the decline of immunity, are expected to exhibit similar effects after surgery, causing excess stress for the body, although it is different from exercise load. The usefulness of cystine/theanine has been investigated in clinical cases and by animal experiments.
Miyachi et al. randomly allocated 33 patients who underwent distal gastrectomy for stomach cancer into two groups: 15 patients to the cystine/theanine administration group (C/T group) and 18 patients to the placebo administration group (control group). The C/T group received oral administration of 700 mg of cystine and 280 mg of theanine for 10 consecutive days (until POD5) from 5 days before surgery including the day of surgery, and the control group received placebo administration [20][13]. The other postoperative management was the same, and time-course blood sampling and REE measurement were performed. Blood interleukin (IL)-6 and CRP reached the maximum levels on the day following surgery, then decreased thereafter, but IL-6 was significantly lower in the C/T group on POD4, and CRP decreased to a significantly low level on POD7, demonstrating recovery. Regarding changes in body temperature, it remained lower in the C/T group than in the control group throughout the course and became significantly lower on POD5. The blood lymphocyte count decreased to approximately 30% of that before surgery on POD1 and recovered thereafter, but recovery was faster in the C/T group, and the difference was significant on POD7 (control group 57.9% vs. C/T group 71.2%). In contrast to the lymphocyte count, the granulocyte count increased to 150% of that before surgery on POD1, then decreased thereafter, and it was significantly lower in the C/T group on POD7 (control group 128.0% vs. C/T group 114.6%). REE increased after surgery. In the control group, it increased by 1.14 times of that before surgery on POD1, gradually decreased thereafter, and became 0.92 times on POD14. On the other hand, in the C/T group, the ratio was 0.99 on POD1, with no increase, and it was significantly different from that in the control group. It slightly increased thereafter, but the increase was inhibited to a maximum level of 1.06 times, observed only on POD5, and the ratio on POD14 was 0.88, being lower than that before surgery. It was clarified that cystine/theanine inhibit the postoperative increase in calorie consumption similar to early enteral nutrition. Changes in the parameters described above also clarified that cystine/theanine administration promotes early recovery after surgery, which led to clarification of the stress-reducing effects. The stress-reducing effects of 1 g of amino acids similar to those of postoperative early enteral nutrition may improve perioperative management in the future.
4. Supportive Data of Animal Experiment for Cystine/Theanine
The effects of cystine/theanine in clinical cases were described above. The usefulness has been also reported by animal experiments using a model simulating gastrointestinal surgery and acute inflammation.
Shibakusa et al. laparotomized mice under anesthesia and prepared a simple laparotomy group in which the abdomen was closed after laparotomy without manipulation, and a small intestine manipulation model in which the small intestine was pulled out of the wound and abrasion with a cotton swab was applied twice to the entire small intestine, and then the wound was closed. The influence of cystine/theanine on postoperative recovery was investigated [33][14]. Using the small intestine manipulation model, 70 mg/kg of cystine/theanine (5:2 mixture) (C/T group) or vehicle (V group) was administered orally for 5 days before surgery, including the day of surgery. The blood IL-6 level markedly increased 2 h after surgery in the V group compared with that in the simple laparotomy group, but the increase was significantly inhibited in the C/T group compared with that in the V group. When GSH contained in the intestinal mucosa and small intestinal Peyer’s patch was measured, it was significantly lower in the V group than that in the simple laparotomy group, but in the C/T group it was maintained at a level similar to that in the simple laparotomy group, and a significant difference from that in the V group was noted. On comparison of the food intake and body weight in the four-postoperative-day period with those before surgery, these were significantly reduced in the V group, whereas the degrees of decrease were small in the C/T group, demonstrating a significant difference from those in the V group. Regarding changes in the body weight, it decreased from that before surgery in the V group, but increased in the C/T group. Regarding the quantity of spontaneous behavior considered to be one of the most important indices of recovery, behavior quantity was significantly suppressed in the V group compared with that in the simple laparotomy group, whereas in the C/T group behavior quantity recovered more rapidly than that in the V group, and a significant difference was noted on day 4. When energy consumption was measured 24 h after surgery using the same model, it decreased immediately after surgery (considered an influence of anesthesia), then increased in the V group, but the increase was significantly inhibited in the C/T group. Based on the above findings, it was confirmed that cystine/theanine administration reduced stress and accelerated recovery. A decrease in the blood and intramuscular GSH levels after surgery in humans has been reported. Demonstration of the effects of cystine/theanine inhibiting the decrease in GSH in the small intestine in the mouse manipulation model suggests that a decrease in GSH was similarly inhibited in clinical surgery [34][15]. Tanaka et al. analyzed the mechanism of the anti-inflammatory effects of cystine after stimulation with LPS using a human monocyte cell line, THP-1 cells [35][16]. They mentioned that after the addition of cystine to cells, cystine was reduced to cysteine in cells after stimulation with LPS and promotion of the production of an anti-inflammatory cytokine, IL-10, accompanying this reduction resulted in a decrease in production of an inflammatory cytokine, IL-6, i.e., anti-inflammatory action. As cysteine generated from cystine in cells may be utilized for GSH synthesis, the anti-inflammatory mechanism of cystine/theanine is outlined in Figure 1. The anti-inflammatory effects of cystine/theanine are exhibited at least through the following two actions: inhibition of stress-induced decrease in the GSH level and reduction of cystine to cysteine in cells. Furthermore, the reduction of the mortality by cystine/theanine administration in a peritonitis model using LPS, which induces severe inflammation, and an intestinal ischemia reperfusion model have been reported, and these studies support the efficacy of cystine/theanine [35,36][16][17]. These clinical studies and animal experiments suggest that cystine/theanine accelerate recovery from stress by preventing a decrease in the GSH level after surgery and inhibiting inflammatory reactions. Therefore, cystine/theanine may be termed stress-reducing amino acids.
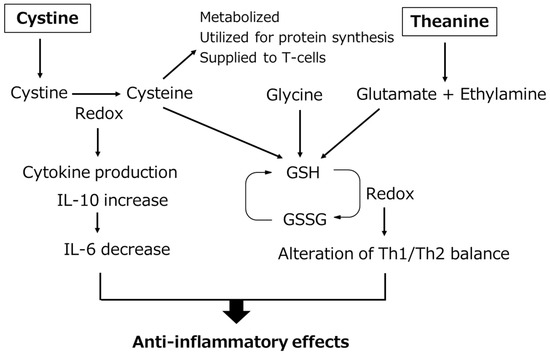
Figure 1. Working hypothesis for the anti-inflammatory effects of cystine/theanine. Cystine/theanine have anti-inflammatory effects both through the maintenance of GSH levels and the promotion of IL-10 production. GSH: glutathione, GSSG: glutathione disulfide, IL-10: interleukin-10, IL-6: interleukin-6, Th1/Th2: type 1 helper T cells/type 2 helper T cells.
References
- U.S. Department of Agriculture Food Data Central. Available online: https://fdc.nal.usda.gov/ (accessed on 15 November 2021).
- Buchanan, J.H. A cystine-rich protein fraction from oxidized alpha-keratin. Biochem. J. 1977, 167, 489–491.
- Rosenberg, L.E.; Crawhall, J.C.; Segal, S. Intestinal transport of cystine and cysteine in man: Evidence for separate mechanisms. J. Clin. Investig. 1967, 46, 30–34.
- Juneja, L.R.; Chu, D.C.; Okubo, T.; Nagato, Y.; Yokogoshi, H. L-Theanine-a unique amino acid of green tea and its relaxation effect in humans. Trens Food Sci. Technol. 1999, 10, 199–204.
- Asatoor, A.M. Tea as a source of urinary ethylamine. Nature 1966, 210, 1358–1360.
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153.
- Rimaniol, A.C.; Mialocq, P.; Clayette, P.; Dormont, D.; Gras, G. Role of glutamate transporters in the regulation of glutathione levels in human macrophages. Am. J. Physiology. Cell Physiol. 2001, 281, C1964–C1970.
- Margonis, K.; Fatouros, I.G.; Jamurtas, A.Z.; Nikolaidis, M.G.; Douroudos, I.; Chatzinikolaou, A.; Mitrakou, A.; Mastorakos, G.; Papassotiriou, I.; Taxildaris, K.; et al. Oxidative stress biomarkers responses to physical overtraining: Implications for diagnosis. Free Radic. Biol. Med. 2007, 43, 901–910.
- Rahman, I.; MacNee, W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000, 16, 534–554.
- Murakami, S.; Kurihara, S.; Titchenal, C.A.; Ohtani, M. Suppression of exercise-induced neutrophilia and lymphopenia in athletes by cystine/theanine intake: A randomized, double-blind, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2010, 7, 23.
- Murakami, S.; Kurihara, S.; Koikawa, N.; Nakamura, A.; Aoki, K.; Yosigi, H.; Sawaki, K.; Ohtani, M. Effects of oral supplementation with cystine and theanine on the immune function of athletes in endurance exercise: Randomized, double-blind, placebo-controlled trial. Biosci. Biotechnol. Biochem. 2009, 73, 817–821.
- Kawada, S.; Kobayashi, K.; Ohtani, M.; Fukusaki, C. Cystine and theanine supplementation restores high-intensity resistance exercise-induced attenuation of natural killer cell activity in well-trained men. J. Strength Cond. Res. 2010, 24, 846–851.
- Miyachi, T.; Tsuchiya, T.; Oyama, A.; Tsuchiya, T.; Abe, N.; Sato, A.; Chiba, Y.; Kurihara, S.; Shibakusa, T.; Mikami, T. Perioperative oral administration of cystine and theanine enhances recovery after distal gastrectomy: A prospective randomized trial. JPEN J. Parenter. Enter. Nutr. 2013, 37, 384–391.
- Shibakusa, T.; Mikami, T.; Kurihara, S.; Chiba, Y.; Tsuchiya, T.; Miyachi, T.; Oyama, A.; Tanaka, K.A.; Koyama, N. Enhancement of postoperative recovery by preoperative oral co-administration of the amino acids, cystine and theanine, in a mouse surgical model. Clin. Nutr. 2012, 31, 555–561.
- Luo, J.L.; Hammarqvist, F.; Andersson, K.; Wernerman, J. Skeletal muscle glutathione after surgical trauma. Ann. Surg. 1996, 223, 420–427.
- Tanaka, K.A.; Kurihara, S.; Shibakusa, T.; Chiba, Y.; Mikami, T. Cystine improves survival rates in a LPS-induced sepsis mouse model. Clin. Nutr. 2015, 34, 1159–1165.
- Miyakuni, T.; Fukatsu, K.; Ri, M.; Murakoshi, S.; Inoue, Y.; Kurihara, S.; Takayama, T.; Yasuhara, H. Cystine and Theanine Improve Survival after Gut Ischemia-Reperfusion. Ann. Nutr. Metab. 2018, 73, 131–137.
More
