Perishable food spoilage caused by fungi is a major cause of discomfort for food producers. Food sensory abnormalities range from aesthetic degeneration to significant aroma, color, or consistency alterations due to this spoilage. Bio-preservation is the use of natural or controlled bacteria or antimicrobials to enhance the quality and safety of food. It has the ability to harmonize and rationalize the required safety requirements with conventional preservation methods and food production safety and quality demands. Even though synthetic preservatives could fix such issues, there is indeed a significant social need for “clean label” foods. As a result, consumers are now seeking foods that are healthier, less processed, and safer. The implementation of antifungal compounds has gotten a lot of attention in recent decades. As a result, the identification and characterization of such antifungal agents has made promising advances.
- anti-fungal
- bio-preservation
- food spoilage
- perishable foods
- shelf life
1. Mode of Action for Various Metabolites
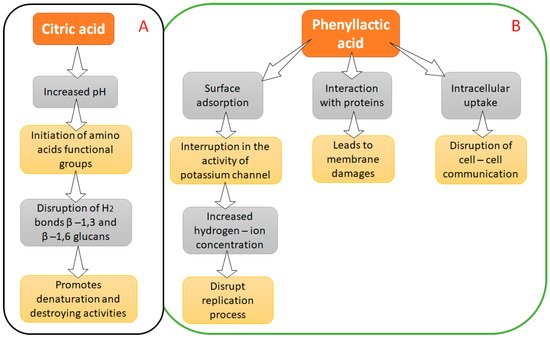
1.1. Citric Acid and Phenyllactic Acid
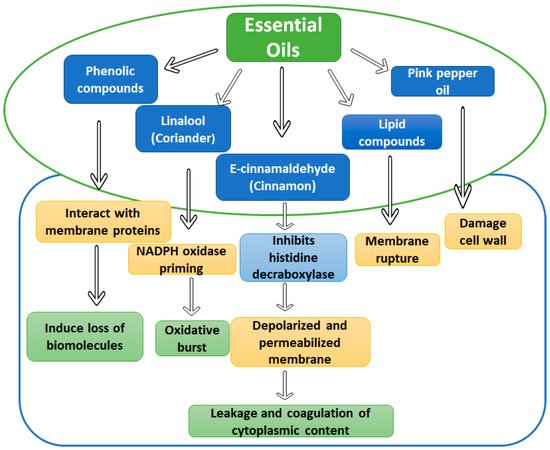
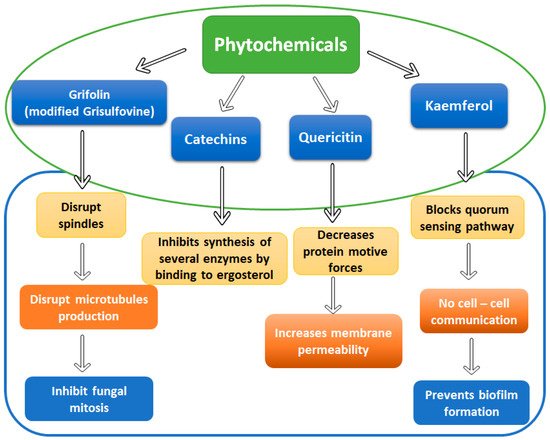
1.2. Essential Oils and Phytochemicals
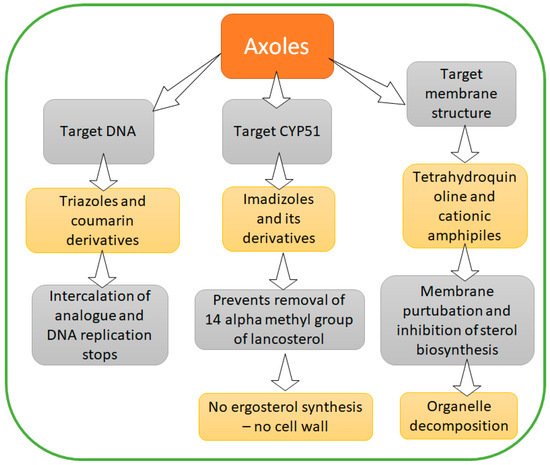
1.3. Azoles
2. Applications Oriented Studies from Laboratory to Pilot Scale
References
- Li, J.; Wang, W.; Xu, S.X.; Magarvey, N.A.; McCormick, J.K. Lactobacillus reuteri-produced cyclic dipeptides quench agr-mediated expression of toxic shock syndrome toxin-1 in staphylococci. Proc. Natl. Acad. Sci. USA 2011, 108, 3360–3365.
- Engelhardt, T.; Albano, H.; Kiskó, G.; Mohácsi-Farkas, C.; Teixeira, P. Antilisterial activity of bacteriocinogenic Pediococcus acidilactici HA6111-2 and Lactobacillus plantarum ESB 202 grown under pH and osmotic stress conditions. Food Microbiol. 2015, 48, 109–115.
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006, 141, 357–366.
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642.
- Rajanikar, R.V.; Nataraj, B.H.; Naithani, H.; Ali, S.A.; Panjagari, N.R.; Behare, P. V Phenyllactic Acid: A green compound for food biopreservation. Food Control 2021, 128, 108184.
- Prema, P.; Smila, D.; Palavesam, A.; Immanuel, G. Production and characterization of an antifungal compound (3-phenyllactic acid) produced by Lactobacillus plantarum strain. Food Bioprocess Technol. 2010, 3, 379–386.
- Kadyan, S.; Pradhan, D. Antifungal Lactic Acid Bacteria (LAB): Potential use in food systems. In Novel Strategies to Improve Shelf-Life and Quality of Foods; Mishra, S.K., Goyal, M.R., Eds.; Apple Academic Press: New York, NY, USA, 2020; pp. 73–94. ISBN 100301027X.
- Svanström, Å.; Boveri, S.; Boström, E.; Melin, P. The lactic acid bacteria metabolite phenyllactic acid inhibits both radial growth and sporulation of filamentous fungi. BMC Res. Notes 2013, 6, 464.
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82.
- Liu, L.-Y.; Li, Z.-H.; Ding, Z.-H.; Dong, Z.-J.; Li, G.-T.; Li, Y.; Liu, J.-K. Meroterpenoid pigments from the basidiomycete Albatrellus ovinus. J. Nat. Prod. 2013, 76, 79–84.
- Gupta, P.; Gupta, S.; Sharma, M.; Kumar, N.; Pruthi, V.; Poluri, K.M. Effectiveness of phytoactive molecules on transcriptional expression, biofilm matrix, and cell wall components of Candida glabrata and its clinical isolates. ACS Omega 2018, 3, 12201–12214.
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464.
- Ivanova, A.; Ivanova, K.; Tzanov, T. Inhibition of quorum-sensing: A new paradigm in controlling bacterial virulence and biofilm formation. In Biotechnological Applications of Quorum Sensing Inhibitors; Kalia, V.C., Ed.; Springer: Singapore, 2018; pp. 3–21.
- Tjia, J.A. Journey into C. albicans Biofilms: Proteomic and Functional Genomic Approaches to Uncovering Mechanisms of Adherence; University of Toronto: Toronto, ON, Canada, 2016.
- Krishnamoorthy, R.; Gassem, M.A.; Athinarayanan, J.; Periyasamy, V.S.; Prasad, S.; Alshatwi, A.A. Antifungal activity of nanoemulsion from Cleome viscosa essential oil against foodborne pathogenic Candida albicans. Saudi J. Biol. Sci. 2021, 28, 286–293.
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55.
- Ali, E.M.; Alkuwayti, M.A.; Aldayel, M.F.; Abdallah, B.M. Coumarin derivative, 5′-hydroxy-auraptene, extracted from Lotus lalambensis, displays antifungal and anti-aflatoxigenic activities against Aspergillus flavus. J. King Saud Univ.-Sci. 2021, 33, 101216.
- Reyes, D.C.; Annis, S.L.; Rivera, S.A.; Leon-Tinoco, A.Y.; Wu, C.; Perkins, L.B.; Perry, J.J.; Ma, Z.X.; Knight, C.W.; Castillo, M.S.; et al. In vitro screening of technical lignins to determine their potential as hay preservatives. J. Dairy Sci. 2020, 103, 6114–6134.
- Dalhoff, A.A.H.; Levy, S.B. Does use of the polyene natamycin as a food preservative jeopardise the clinical efficacy of amphotericin B? A word of concern. Int. J. Antimicrob. Agents 2015, 45, 564–567.
- Nionelli, L.; Wang, Y.; Pontonio, E.; Immonen, M.; Rizzello, C.G.; Maina, H.N.; Katina, K.; Coda, R. Antifungal effect of bioprocessed surplus bread as ingredient for bread-making: Identification of active compounds and impact on shelf-life. Food Control 2020, 118, 107437.
- Bukvicki, D.; Giweli, A.; Stojkovic, D.; Vujisic, L.; Tesevic, V.; Nikolic, M.; Sokovic, M.; Marin, P.D. Cheese supplemented with Thymus algeriensis oil, a potential natural food preservative. J. Dairy Sci. 2018, 101, 3859–3865.
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A.; Asker, D.; Awad, T.S. Characterization of antifungal metabolites produced by novel lactic acid bacterium and their potential application as food biopreservatives. Ann. Agric. Sci. 2019, 64, 71–78.
- Nebbia, S.; Lamberti, C.; Lo Bianco, G.; Cirrincione, S.; Laroute, V.; Cocaign-Bousquet, M.; Cavallarin, L.; Giuffrida, M.G.; Pessione, E. Antimicrobial potential of food Lactic Acid Bacteria: Bioactive peptide decrypting from caseins and bacteriocin production. Microorganisms 2021, 9, 65.
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A. Novel antifungal bacteriocin from Lactobacillus paracasei KC39 with anti-mycotoxigenic properties. Biosci. Res. 2018, 15, 4171–4183.
- Ahmad Rather, I.; Seo, B.J.; Rejish Kumar, V.J.; Choi, U.; Choi, K.; Lim, J.H.; Park, Y. Isolation and characterization of a proteinaceous antifungal compound from Lactobacillus plantarum YML 007 and its application as a food preservative. Lett. Appl. Microbiol. 2013, 57, 69–76.
- Yang, E.J.; Chang, H.C. Purification of a new antifungal compound produced by Lactobacillus plantarum AF1 isolated from kimchi. Int. J. Food Microbiol. 2010, 139, 56–63.
- Ryu, E.H.; Yang, E.J.; Woo, E.R.; Chang, H.C. Purification and characterization of antifungal compounds from Lactobacillus plantarum HD1 isolated from kimchi. Food Microbiol. 2014, 41, 19–26.
- Badr, A.N.; Abdel-Fatah, S.M.; Sree, Y.H.A.; Amra, H.A. Mycotoxigenic fungi and mycotoxins in Egyptian barley under climate changes. Res. J. Environ. Toxicol. 2017, 11, 1–10.
- Badr, A.N.; Nada, F.; Shehata, M.G.; Amra, H.A. Anti-mycotic and anti-mycotoxigenic properties of Egyptian dill. J. Appl. Sci. 2017, 17, 184–195.
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78.
