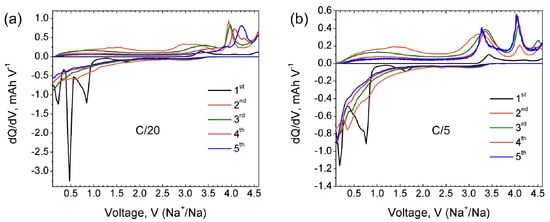
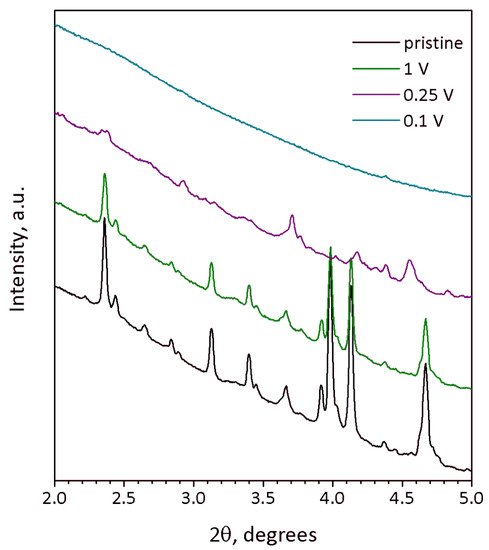
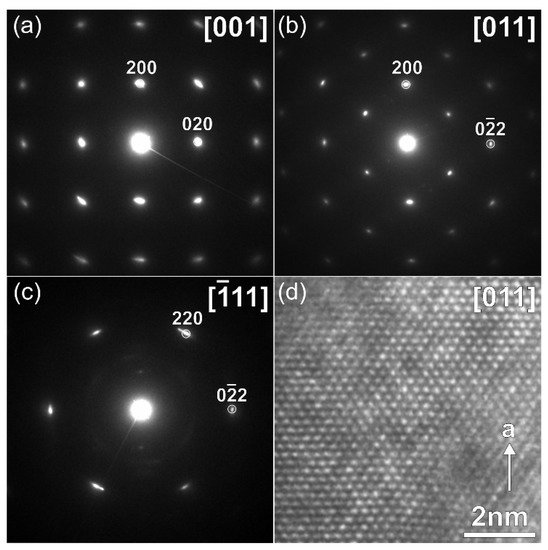
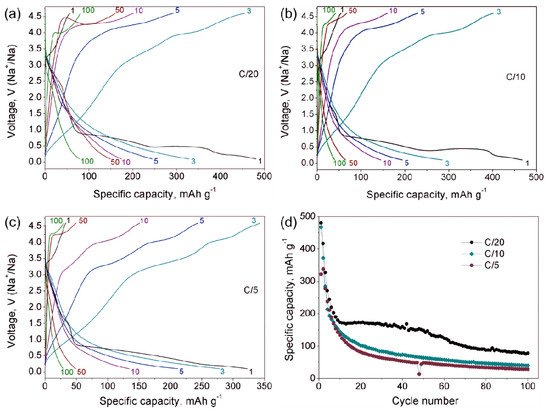
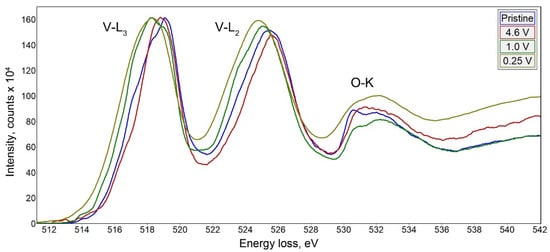
Na9V14O35 (η-NaxV2O5) has been synthesized by a solid-state route in an evacuated sealed silica tube and tested as electroactive material for Na half-cells. Being charged to 4.6 V vs. Na+/Na, almost 3 Na can be extracted per Na9V14O35 formula unit, resulting in a charge capacity of about 60 mAh g−1. Upon discharge below 1 V, Na9V14O35 uptakes Na up to the Na:V = 1:1 atomic ratio that is accompanied by a drastic increase of the separation between the layers of the VO4 tetrahedra and VO5 tetragonal pyramids, and a volume increase of about 31%. The induced structure instability triggers a transformation of the ordered layered Na9V14O35 structure into a rock-salt type disordered structure. Ultimately, the amorphous products of a conversion reaction are formed at 0.1 V, delivering the discharge capacity up to 490 mAh g−1, which, however, quickly fades with the number of charge-discharge cycles.
- Na-ion batteries
- sodium-vanadium bronzes
- electrochemical cycling
1. Introduction
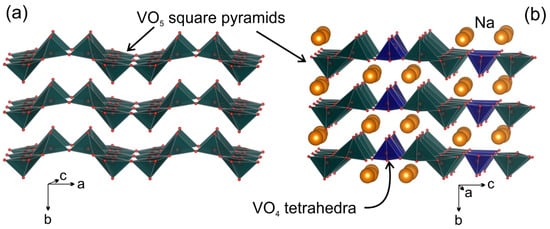
2. Current Insights
References
- Masquelier, C.; Croguennec, L.; Polyanionic (phosphates, silicates, sulfates) frameworks as electrode materials for rechargeable Li (or Na) batteries. Chem. Rev. 2013,, 113,, 6552.Masquelier, C.; Croguennec, L. Polyanionic (phosphates, silicates, sulfates) frameworks as electrode materials for rechargeable Li (or Na) batteries. Chem. Rev. 2013, 113, 6552–6591.
- Naoaki Yabuuchi; Kei Kubota; Mouad Dahbi; Shinichi Komaba; Research Development on Sodium-Ion Batteries. Chemical Reviews 2014, 114, 11636-11682, 10.1021/cr500192f.Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682.
- Keld West; Sodium insertion in vanadium oxides. Solid State Ionics 1988, 28-30, 1128-1131, 10.1016/0167-2738(88)90343-8.West, K.; Zachau-Christiansen, B.; Jacobsen, T.; Skaarup, S. Sodium insertion in vanadium oxides. Solid State Ion. 1988, 28−30, 1128–1131.
- Pouchard, M.; Casalot, A.; Galy, J.; Hagenmuller, P; Vanadium bronzes with NaxV2O5 formula. Bull. Soc. Chim. Fr. 1967, 11, 4343.Pouchard, M.; Casalot, A.; Galy, J.; Hagenmuller, P. Vanadium bronzes with NaxV2O5 formula. Bull. Soc. Chim. Fr. 1967, 11, 4343–4348.
- Y. Kanke; E. Takayama-Muromachi; K. Kato; Y. Matsui; Phase equilibrium study of the system NaV2O5V2O3V2O5 at 923 K. Journal of Solid State Chemistry 1990, 89, 130-137, 10.1016/0022-4596(90)90302-e.Kanke, Y.; Takayama-Muromachi, E.; Kato, K.; Matsui, Y. Phase equilibrium study of the system NaV2O5–V2O3–V2O5 at 923 K. J. Solid State Chem. 1990, 89, 130–137.
- Jean-Michel Savariault; Jean-Luc Parize; Danielle Ballivet-Tkatchendo; Jean Galy; τ-NaxV2O5(x= 0.64): A Vanadium Bronze with an Original Intergrowth Structure. Journal of Solid State Chemistry 1996, 122, 1-6, 10.1006/jssc.1996.0072.Savariault, J.M.; Parize, J.L.; Tkatchenko, D.B.; Galy, V. τ-NaxV2O5(x = 0.64): A vanadium bronze with an original intergrowth structure. J. Solid State Chem. 1996, 122, 1–6.
- Marianne Safrany Renard; Nicolas Emery; Evgenii M. Roginskii; Rita Baddour-Hadjean; Jean-Pierre Pereira-Ramos; Crystal structure determination of a new sodium vanadium bronze electrochemically formed. Journal of Solid State Chemistry 2017, 254, 62-68, 10.1016/j.jssc.2017.07.012.Renard, M.S.; Emery, N.; Roginskii, E.M.; Baddour-Hadjeana, R.; Pereira-Ramos, J.-P. Crystal structure determination of a new sodium vanadium bronze electrochemically formed. J. Solid State Chem. 2017, 254, 62–68.
- A. D. Wadsley; The crystal structure of Na2−xV6O15. Acta Crystallographica 1955, 8, 695-701, 10.1107/s0365110x55002144.Wadsley, A.D. The crystal structure of Na2−xV6O15. Acta Cryst. 1955, 8, 695–701.
- Nicolas Emery; Rita Baddour-Hadjean; Dauren Batyrbekuly; Barbara Laïk; Zhumabay Bakenov; Jean-Pierre Pereira-Ramos; γ-Na0.96V2O5: A New Competitive Cathode Material for Sodium-Ion Batteries Synthesized by a Soft Chemistry Route. Chemistry of Materials 2018, 30, 5305-5314, 10.1021/acs.chemmater.8b02066.Emery, N.; Baddour-Hadjean, R.; Batyrbekuly, D.; Laϊk, B.; Bakenov, Z.; Pereira-Ramos, J.-P. γ-Na0.96V2O5: A new competitive cathode material for sodium ion battery synthesized by a soft chemistry route. Chem. Mater. 2018, 30, 5305–5314.
- A. Carpy; J. Galy; Affinement de la structure cristalline du bronze NaV2O5α'. Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 1975, 31, 1481-1482, 10.1107/s0567740875005468.Carpy, A.; Galy, J. Affinement de la structure cristalline du bronze NaV2O5α’. Acta Crystallogr. 1975, B31, 1481–1482.
- Ganganagappa Nagaraju; Gujjarahalli Thimmanna Chandrappa; Solution phase synthesis of Na0.28V2O5 nanobelts into nanorings and the electrochemical performance in Li battery. Materials Research Bulletin 2012, 47, 3216-3223, 10.1016/j.materresbull.2012.08.010.Nagaraju, G.; Chandrappa, G.T. Solution phase synthesis of Na0.28V2O5 nanobelts into nanorings and the electrochemical performance in Li battery. Mater. Res. Bull. 2012, 47, 3216–3223.
- L. Znaidi; N. Baffier; M. Huber; Synthesis of vanadium bronzes MxV2O5 through sol-gel processes I - Monoclinic bronzes (M = Na, Ag). Materials Research Bulletin 1989, 24, 1501-1514, 10.1016/0025-5408(89)90161-x.Znaidi, L.; Baffier, N.; Huber, M. Synthesis of vanadium bronzes NaxV2O5 through sol–gel processes I-monoclinic bronzes (M = Na, Ag). Mater. Res. Bull. 1989, 24, 1501–1514.
- Inseok Seo; Gil Chan Hwang; Jae-Kwang Kim; YoungSik Kim; Electrochemical characterization of micro-rod β-Na0.33V2O5 for high performance lithium ion batteries. Electrochimica Acta 2016, 193, 160-165, 10.1016/j.electacta.2016.02.026.Seo, I.; Hwang, G.C.; Kim, J.-K.; Kim, Y. Electrochemical characterization of micro-rod β-Na0.33V2O5 for high performance lithium ion batteries. Electrochim. Acta 2016, 193, 160–165.
- Pan-Pan Wang; Cheng-Yan Xu; Fei-Xiang Ma; Li Yang; Liang Zhen; In situ soft-chemistry synthesis of β-Na0.33V2O5 nanorods as high-performance cathode for lithium-ion batteries. RSC Advances 2016, 6, 105833-105839, 10.1039/c6ra23484d.Wang, P.-P.; Xu, C.-Y.; Ma, F.-X.; Yang, L.; Zhen, L. In situ soft-chemistry synthesis of β-Na0.33V2O5 nanorods as high-performance cathode for lithium-ion batteries. RSC Adv. 2016, 6, 105833–105839.
- Shuquan Liang; Jiang Zhou; Guozhao Fang; Cheng Zhang; Jun Wu; Yan Tang; Anqiang Pan; Synthesis of mesoporous β-Na0.33V2O5 with enhanced electrochemical performance for lithium ion batteries. Electrochimica Acta 2014, 130, 119-126, 10.1016/j.electacta.2014.02.131.Liang, S.Q.; Zhou, J.; Fang, G.Z.; Zhang, C.; Wu, J.; Tang, Y.; Pan, A.Q. Synthesis of mesoporous β-Na0.33V2O5 with enhanced electrochemical performance for lithium ion batteries. Electrochim. Acta 2014, 130, 119–126.
- Yakun Lu; Jun Wu; Jun Liu; Ming Lei; Shasha Tang; Peijie Lu; Linyu Yang; Haoran Yang; Qian Yang; Facile Synthesis of Na0.33V2O5 Nanosheet-Graphene Hybrids as Ultrahigh Performance Cathode Materials for Lithium Ion Batteries. ACS Applied Materials & Interfaces 2015, 7, 17433-17440, 10.1021/acsami.5b04827.Lu, Y.K.; Wu, J.; Liu, J.; Lei, M.; Tang, S.S.; Lu, P.J.; Yang, L.Y.; Yang, H.R.; Yang, Q. Facile synthesis of Na0.33V2O5 nanosheet-graphene hybrids as ultrahigh performance cathode materials for lithium ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 17433–17440.
- C. Delmas; H. Cognac-Auradou; J.M. Cocciantelli; M. Ménétrier; J.P. Doumerc; The LixV2O5 system: An overview of the structure modifications induced by the lithium intercalation. Solid State Ionics 1994, 69, 257-264, 10.1016/0167-2738(94)90414-6.Delmas, C.; Cognac-Auradou, H.; Cocciantelli, J.M.; Ménétrier, M.; Doumerc, J.P. The LixV2O5 system: An overview of the structure modifications induced by the lithium intercalation. Solid State Ion. 1994, 69, 257–264.
- Haodong Liu; Zhuoying Zhu; Qizhang Yan; Sicen Yu; Xin He; Yan Chen; Rui Zhang; Lu Ma; Tongchao Liu; Matthew Li; et al.Ruoqian LinYiming ChenYejing LiXing XingYoonjung ChoiLucy GaoHelen Sung-Yun ChoKe AnJun FengRobert KosteckiKhalil AmineTianpin WuJun LuHuolin L. XinShyue Ping OngPing Liu A disordered rock salt anode for fast-charging lithium-ion batteries. Nature 2020, 585, 63-67, 10.1038/s41586-020-2637-6.Liu, H.; Zhu, Z.; Yan, Q.; Yu, S.; He, X.; Chen, Y.; Zhang, R.; Ma, L.; Liu, T. A disordered rock salt anode for fast-charging lithium-ion batteries. Nature 2020, 585, 63–67.
- D. Muller-Bouvet; R. Baddour-Hadjean; M. Tanabe; Le Thanh Nguyen Huynh; M.L.P. Le; J.P. Pereira-Ramos; Electrochemically formed α’-NaV2O5: A new sodium intercalation compound. Electrochimica Acta 2015, 176, 586-593, 10.1016/j.electacta.2015.07.030.Muller-Bouvet, D.; Baddour-Hadjean, R.; Tanabe, M.; Huynh, L.T.N.; Le, M.L.P.; Pereira-Ramos, J.-P. Electrochemically formed α’-NaV2O5: A new sodium intercalation compound. Electrochim. Acta 2015, 176, 586–593.
- Sanja Tepavcevic; Hui Xiong; Vojislav R. Stamenkovic; Xiaobing Zuo; Mahalingam Balasubramanian; Vitali B. Prakapenka; Christopher S. Johnson; Tijana Rajh; Nanostructured Bilayered Vanadium Oxide Electrodes for Rechargeable Sodium-Ion Batteries. ACS Nano 2011, 6, 530-538, 10.1021/nn203869a.Tepavcevic, S.; Xiong, H.; Stamenkovic, V.R.; Zuo, X.; Balasubramanian, M.; Prakapenka, V.B.; Johnson, C.S.; Rajh, T. Nanostructured Bilayered Vanadium Oxide Electrodes for Rechargeable Sodium-Ion Batteries. ACS Nano 2012, 6, 530–538.
- Fang Hu; Wei Jiang; Yidi Dong; Xiaoyong Lai; Li Xiao; Xiang Wu; Synthesis and electrochemical performance of NaV6O15 microflowers for lithium and sodium ion batteries. RSC Advances 2017, 7, 29481-29488, 10.1039/c7ra04388k.Hu, F.; Jiang, W.; Dong, Y.; Lai, X.; Xiao, L.; Wu, X. Synthesis and electrochemical performance of NaV6O15 microflowers for lithium and sodium ion batteries. RSC Adv. 2017, 7, 29481–29488.
- P. Millet; J.-Y. Henry; J. Galy; The vanadium oxide bronze η-NaxV2O5(x= 1.286). Acta Crystallographica Section C Crystal Structure Communications 1999, 55, 276-279, 10.1107/s010827019801587x.Millet, P.; Henry, J.-Y.; Galy, J. The vanadium oxide bronze η-NaxV2O5 (x = 1.286). Acta Cryst. 1999, C55, 276–279.
- Masahiko Isobe; Yutaka Ueda; Yoshio Oka; Takeshi Yao; Crystal Structure and Magnetic Properties of Na9V14O35:Sodium–Vanadium Bronze η-NaxV2O5. Journal of Solid State Chemistry 1999, 145, 361-365, 10.1006/jssc.1999.8282.Isobe, M.; Ueda, Y.; Oka, Y.; Yao, T. Crystal structure and magnetic properties of Na9V14O35: Sodium-vanadium bronze η-NaxV2O5. J. Solid State Chem. 1999, 145, 361–365.
- R. D. Shannon; Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A 1976, 32, 751-767, 10.1107/s0567739476001551.Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767.
- Haiyan Tan; Jo Verbeeck; Artem Abakumov; Gustaaf Van Tendeloo; Oxidation state and chemical shift investigation in transition metal oxides by EELS. Ultramicroscopy 2012, 116, 24-33, 10.1016/j.ultramic.2012.03.002.Tan, H.; Verbeeck, J.; Abakumov, A.; Van Tendeloo, G. Oxidation state and chemical shift investigation in transition metal oxides by EELS. Ultramicroscopy 2012, 116, 24–33.
