Nicotinamide adenine dinucleotide (NAD+) is an essential molecule involved in various metabolic reactions, acting as an electron donor in the electron transport chain and as a co-factor for NAD+-dependent enzymes. Despite systematic claims of overall decline in NAD+ levels with aging in multiple species, including humans, the evidence to support such claims is very limited and often restricted to a single tissue or cell type. The literature on the topic has been reviewed and it is found that there is a need for much larger, preferably longitudinal, studies to assess how NAD+ levels develop with aging.
- NAD+
- aging
- yeast
- C. elegans
- rat
- monkey
- human
1. Introduction
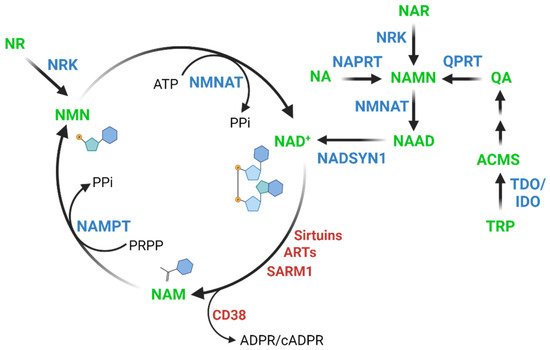
2. Relationship between NAD+ Levels and Aging across Species
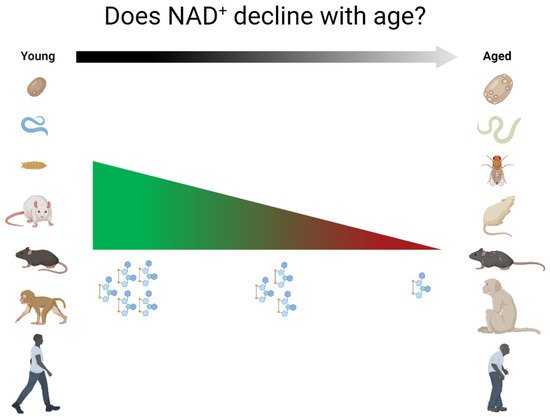
2.1. Non-Mammalian Species
2.1.1. Yeast
2.1.2. Caenorhabditis Elegans
Only two studies have reported on NAD+ levels in aging C. Elegans. NAD+ levels were found to be reduced in aged C. elegans (day 17 [15] and day 8 [16], respectively) compared to young controls (day 1). These studies indicated an association between NAD+ and lifespan, and many subsequent studies in C. elegans have focused on the role of NAD+ consuming enzymes or supplementation with NAD+ precursors to affect longevity [16][17][18].2.1.3. Drosophila Melanogaster
It is impossible to identify any papers that address whether levels of NAD+ decline with age in this model, despite the ease with which such experiments could be performed.
2.2. Rodents
2.2.1. Rats
2.2.2. Mice
The most comprehensive study to date on the development of NAD+ levels with aging in mice, studied several tissues [23]. It showed that aged mice had a decrease in NAD+ in only 10 out of 21 tested tissues. In brown and two different white adipose tissues (retroperitoneal and inguinal), as well as in the jejunum, they observed a ~40–50% NAD+ reduction with age, whereas in gastrocnemius, soleus, quadriceps, liver, kidney, and descending colon NAD+ levels were reduced by ~10–20%. The NAD+ levels in the remaining 11 tissues including heart, brain, spleen, lung, pancreas, gonadal white adipose tissue, and multiple parts of the gastrointestinal tract were found to be unchanged by aging. It is clear from this study alone that the apparent consensus that NAD+ levels universally decline with aging is inaccurate. Overall, mice have been studied more extensively in this regard and there is quite reliable evidence of NAD+ decline in aging skeletal muscle [5][15][23][24][25][26][27], some adipose tissues [23][24][25] and hippocampal areas of the brain [28][29][30]. However, there are conflicting reports on liver [15][24][31][32] and effects of age on NAD+ levels in heart, lung, spleen, pancreas, and intestine remain relatively uninvestigated.2.3. Primates
2.3.1. Monkeys
2.3.2. Humans
The literature on the association between human NAD+ and ageing presents us with very little data on peripheral tissues. There is a single study showing marked reduction in skin NAD+ levels with age [35], as well as one showing an age-associated 30% reduction in NAD+ in liver from patients with hepatocellular carcinoma [36]. No study has investigated the effect of age on adipose NAD+ levels at all, and the data on skeletal muscle is limited to a single report on BioRxiv [37] and was removed before peer-reviewed publication [38].3. Conclusions and perspective
There are remarkably few studies that assess NAD+ levels with aging. This is true for most of the commonly used model organisms as well as for humans. Moreover, even within specific tissues, there are discrepancies in the literature, and many tissues in multiple organisms have only been investigated by a single research group or not at all. Thus, there is considerable disagreement between what the field assumes to know on the topic of NAD+ in aging and what is scientifically supported. This poor-founded perpetuation of the idea that NAD+ levels universally decrease with age is misleading, and it may lead to the loss of important nuances in our collective understanding of NAD metabolism. There is a need for longitudinal studies investigating the way NAD+ levels behave in various tissues during aging in various model organisms, and much larger cross-sectional studies in humans are required to address this specific question.
References
- Katrina L Bogan; Charles Brenner; Nicotinic Acid, Nicotinamide, and Nicotinamide Riboside: A Molecular Evaluation of NAD+ Precursor Vitamins in Human Nutrition. Annual Review of Nutrition 2008, 28, 115-130, 10.1146/annurev.nutr.28.061807.155443.
- Anna Wilk; Faisal Hayat; Richard Cunningham; Jianfeng Li; Silvia Garavaglia; Leila Zamani; Davide M. Ferraris; Peter Sykora; Joel Andrews; Jennifer Clark; et al.Amanda DavisLaurent ChaloinMenico RizziMarie MigaudRobert W. Sobol Extracellular NAD+ enhances PARP-dependent DNA repair capacity independently of CD73 activity. Scientific Reports 2020, 10, 1-21, 10.1038/s41598-020-57506-9.
- Michael B. Schultz; David A. Sinclair; Why NAD + Declines during Aging: It’s Destroyed. Cell Metabolism 2016, 23, 965-966, 10.1016/j.cmet.2016.05.022.
- Sofie Lautrup; David A. Sinclair; Mark P. Mattson; Evandro F. Fang; NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metabolism 2019, 30, 630-655, 10.1016/j.cmet.2019.09.001.
- Hongbo Zhang; Dongryeol Ryu; Yibo Wu; Karim Gariani; Xu Wang; Peiling Luan; Davide D’Amico; Eduardo R. Ropelle; Matthias P. Lutolf; Ruedi Aebersold; et al.Kristina SchoonjansKeir J. MenziesJohan Auwerx NAD + repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 2016, 352, 1436-1443, 10.1126/science.aaf2693.
- Keisuke Okabe; Keisuke Yaku; Kazuyuki Tobe; Takashi Nakagawa; Implications of altered NAD metabolism in metabolic disorders. Journal of Biomedical Science 2019, 26, 34, 10.1186/s12929-019-0527-8.
- Liang-Jun Yan; Jinzi Wu; Zhen Jin; Hong Zheng; Sources and implications of NADH/NAD+ redox imbalance in diabetes and its complications. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 2016, ume 9, 145-153, 10.2147/dmso.s106087.
- Lan Fan; Jose M. Cacicedo; Yasuo Ido; Impaired nicotinamide adenine dinucleotide (NAD+) metabolism in diabetes and diabetic tissues: Implications for nicotinamide‐related compound treatment. Journal of Diabetes Investigation 2020, 11, 1403-1419, 10.1111/jdi.13303.
- Aaron N Long; Katrina Owens; Anna E Schlappal; Tibor Kristian; Paul S Fishman; Rosemary A Schuh; Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC Neurology 2015, 15, 1-14, 10.1186/s12883-015-0272-x.
- Chongkui Sun; Kun Wang; Amanda J Stock; Yi Gong; Tyler G Demarest; Beimeng Yang; Neelam Giri; Lea Harrington; Blanche P Alter; Sharon A Savage; et al.Vilhelm A BohrYie Liu Re‐equilibration of imbalanced NAD metabolism ameliorates the impact of telomere dysfunction. The EMBO Journal 2020, 39, e103420, 10.15252/embj.2019103420.
- Valter D. Longo; Gerald S. Shadel; Matt Kaeberlein; Brian Kennedy; Replicative and Chronological Aging in Saccharomyces cerevisiae. Cell Metabolism 2012, 16, 18-31, 10.1016/j.cmet.2012.06.002.
- Stephen S. Lin; Jill K. Manchester; Jeffrey I. Gordon; Enhanced Gluconeogenesis and Increased Energy Storage as Hallmarks of Aging in Saccharomyces cerevisiae. Journal of Biological Chemistry 2001, 276, 36000-36007, 10.1074/jbc.m103509200.
- Anthony O. Beas; Patricia B. Gordon; Clara L. Prentiss; Carissa Perez Olsen; Matthew A. Kukurugya; Bryson D. Bennett; Susan M. Parkhurst; Daniel E. Gottschling; Independent regulation of age associated fat accumulation and longevity. Nature Communications 2020, 11, 1-10, 10.1038/s41467-020-16358-7.
- Ivan Orlandi; Giulia Stamerra; Marina Vai; Altered Expression of Mitochondrial NAD+ Carriers Influences Yeast Chronological Lifespan by Modulating Cytosolic and Mitochondrial Metabolism. Frontiers in Genetics 2018, 9, 676, 10.3389/fgene.2018.00676.
- Laurent Mouchiroud; Riekelt Houtkooper; Norman Moullan; Elena Katsyuba; Dongryeol Ryu; Carles Canto; Adrienne Mottis; Young Suk Jo; Mohan Viswanathan; Kristina Schoonjans; et al.Leonard GuarenteJohan Auwerx The NAD+/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 2013, 154, 430-441, 10.1016/j.cell.2013.06.016.
- Evandro Fei Fang; Morten Scheibye-Knudsen; Lear Brace; Henok Kassahun; Tanima SenGupta; Hilde Nilsen; James R. Mitchell; Deborah L. Croteau; Vilhelm A. Bohr; Defective Mitophagy in XPA via PARP-1 Hyperactivation and NAD+/SIRT1 Reduction. Cell 2014, 157, 882-896, 10.1016/j.cell.2014.03.026.
- Evandro Fei Fang; Henok Kassahun; Deborah L. Croteau; Morten Scheibye-Knudsen; Krisztina Marosi; Huiming Lu; Raghavendra A. Shamanna; Sumana Kalyanasundaram; Ravi Chand Bollineni; Mark A. Wilson; et al.Wendy B. IserBradley N. WollmanMarya MorevatiJun LiJesse S. KerrQiping LuTyler B. WaltzJane TianDavid A. SinclairMark P. MattsonHilde NilsenVilhelm A. Bohr NAD + Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metabolism 2016, 24, 566-581, 10.1016/j.cmet.2016.09.004.
- Dongryeol Ryu; Hongbo Zhang; Eduardo R. Ropelle; Vincenzo Sorrentino; Davi A. G. Mázala; Laurent Mouchiroud; Philip L. Marshall; Matthew D. Campbell; Amir Safi Ali; Gary M. Knowels; et al.Stéphanie BelleminShama R. IyerXu WangKarim GarianiAnthony A. SauveCarles CantóKevin E. ConleyLudivine WalterRichard M. LoveringEva R. ChinBernard J. JasminDavid J. MarcinekKeir J. MenziesJohan Auwerx NAD + repletion improves muscle function in muscular dystrophy and counters global PARylation. Science Translational Medicine 2016, 8, 361ra139-361ra139, 10.1126/scitranslmed.aaf5504.
- Nady Braidy; Gilles Guillemin; Hussein Mansour; Tailoi Chan-Ling; Anne Poljak; Ross Grant; Age Related Changes in NAD+ Metabolism Oxidative Stress and Sirt1 Activity in Wistar Rats. PLoS ONE 2011, 6, e19194, 10.1371/journal.pone.0019194.
- Nady Braidy; Anne Poljak; Ross Grant; Tharusha Jayasena; Hussein Mansour; Tailoi Chan-Ling; Gilles J. Guillemin; George Smythe; Perminder Sachdev; Mapping NAD+ metabolism in the brain of ageing Wistar rats: potential targets for influencing brain senescence. Biogerontology 2013, 15, 177-198, 10.1007/s10522-013-9489-5.
- Cao Ma; Chenchen Pi; Yue Yang; Lin Lin; Yingai Shi; Yan Li; Yulin Li; Xu He; Nampt Expression Decreases Age-Related Senescence in Rat Bone Marrow Mesenchymal Stem Cells by Targeting Sirt1. PLoS ONE 2017, 12, e0170930, 10.1371/journal.pone.0170930.
- Chenchen Pi; Yue Yang; Yanan Sun; Huan Wang; Hui Sun; Mao Ma; Lin Lin; Yingai Shi; Yan Li; Yulin Li; et al.Xu He Nicotinamide phosphoribosyltransferase postpones rat bone marrow mesenchymal stem cell senescence by mediating NAD+–Sirt1 signaling. Aging 2019, 11, 3505-3522, 10.18632/aging.101993.
- Melanie R. McReynolds; Karthikeyani Chellappa; Eric Chiles; Connor Jankowski; Yihui Shen; Li Chen; Hélène C. Descamps; Sarmistha Mukherjee; Yashaswini R. Bhat; Siddharth R. Lingala; et al.Qingwei ChuPaul BotolinFaisal HayatTomohito DokeKatalin SusztakChristoph A. ThaissWenyun LuMarie E. MigaudXiaoyang SuJoshua D. RabinowitzJoseph A. Baur NAD+ flux is maintained in aged mice despite lower tissue concentrations. Cell Systems 2021, 12, 1160-1172.e4, 10.1016/j.cels.2021.09.001.
- Jun Yoshino; Kathryn F. Mills; Myeong Jin Yoon; Shin-Ichiro Imai; Nicotinamide Mononucleotide, a Key NAD+ Intermediate, Treats the Pathophysiology of Diet- and Age-Induced Diabetes in Mice. Cell Metabolism 2011, 14, 528-536, 10.1016/j.cmet.2011.08.014.
- Ling-Fang Wang; Lianjie Miao; Xiao-Lei Wang; Cong-Cong Huang; Yi-Song Qian; Xuan Huang; Wan-Zhu Jin; Miao Lian-Jie; Mingui Fu; Ke-Yu Deng; et al.Hong-Bo Xin CD38 deficiency suppresses adipogenesis and lipogenesis in adipose tissues through activating Sirt1/PPARγ signaling pathway. Journal of Cellular and Molecular Medicine 2017, 22, 101-110, 10.1111/jcmm.13297.
- Ana P. Gomes; Nathan Price; Alvin J.Y. Ling; Javid J. Moslehi; Magdalene Montgomery; Luis Rajman; James P. White; João Teodoro; Christiane D. Wrann; Basil Hubbard; et al.Evi M. MerckenCarlos PalmeiraRafael de CaboAnabela RoloNigel TurnerEric L. BellDavid A. Sinclair Declining NAD+ Induces a Pseudohypoxic State Disrupting Nuclear-Mitochondrial Communication during Aging. Cell 2013, 155, 1624-1638, 10.1016/j.cell.2013.11.037.
- David W. Frederick; Emanuele Loro; Ling Liu; Antonio Davila; Karthikeyani Chellappa; Ian Silverman; William J. Quinn; Sager J. Gosai; Elisia D. Tichy; James G. Davis; et al.Foteini MourkiotiBrian D. GregoryRyan W. DellingerPhilip RedpathMarie E. MigaudEiko Nakamaru-OgisoJoshua D. RabinowitzTejvir S. KhuranaJoseph A. Baur Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell Metabolism 2016, 24, 269-282, 10.1016/j.cmet.2016.07.005.
- Liana R Stein; Shin‐Ichiro Imai; Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. The EMBO Journal 2014, 33, 1321-1340, 10.1002/embj.201386917.
- Sean Johnson; David F. Wozniak; S. Imai; CA1 Nampt knockdown recapitulates hippocampal cognitive phenotypes in old mice which nicotinamide mononucleotide improves. npj Aging and Mechanisms of Disease 2018, 4, 1-12, 10.1038/s41514-018-0029-z.
- Stephen A. Martin; Tyler M. DeMuth; Karl N. Miller; Thomas D. Pugh; Michael A. Polewski; Ricki J. Colman; Kevin Eliceiri; Timothy Mark Beasley; Sterling C. Johnson; Rozalyn M. Anderson; et al. Regional metabolic heterogeneity of the hippocampus is nonuniformly impacted by age and caloric restriction. Aging Cell 2015, 15, 100-110, 10.1111/acel.12418.
- Morten Dall; Melanie Penke; Karolina Sulek; Madlen Matz-Soja; Birgitte Holst; Antje Garten; Wieland Kiess; Jonas T. Treebak; Hepatic NAD+ levels and NAMPT abundance are unaffected during prolonged high-fat diet consumption in C57BL/6JBomTac mice. Molecular and Cellular Endocrinology 2018, 473, 245-256, 10.1016/j.mce.2018.01.025.
- Morten Dall; Samuel A. J. Trammell; Magnus Asping; Anna S. Hassing; Marianne Agerholm; Sara G. Vienberg; Matthew P. Gillum; Steen Larsen; Jonas T. Treebak; Mitochondrial function in liver cells is resistant to perturbations in NAD+ salvage capacity. Journal of Biological Chemistry 2019, 294, 13304-13326, 10.1074/jbc.ra118.006756.
- Kenneth L. Chiou; Michael J. Montague; Elisabeth A. Goldman; Marina M. Watowich; Sierra N. Sams; Jeff Song; Julie E. Horvath; Kirstin N. Sterner; Angelina V. Ruiz-Lambides; Melween I. Martínez; et al.James P. HighamLauren J. N. BrentMichael L. PlattNoah Snyder-Mackler Rhesus macaques as a tractable physiological model of human ageing. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 2020, 375, 20190612, 10.1098/rstb.2019.0612.
- Thomas D. Pugh; Matthew W. Conklin; Trent D. Evans; Michael A. Polewski; Hannah J. Barbian; Rachelle Pass; Bradley D. Anderson; Ricki J. Colman; Kevin W. Eliceiri; Patricia J. Keely; et al.Richard WeindruchT. Mark BeasleyRozalyn M. Anderson A shift in energy metabolism anticipates the onset of sarcopenia in rhesus monkeys. Aging Cell 2013, 12, 672-681, 10.1111/acel.12091.
- Hassina Massudi; Ross Grant; Nady Braidy; Jade Guest; Bruce Farnsworth; Gilles Guillemin; Age-Associated Changes In Oxidative Stress and NAD+ Metabolism In Human Tissue. PLoS ONE 2012, 7, e42357, 10.1371/journal.pone.0042357.
- Can‐Can Zhou; Xi Yang; Xia Hua; Jian Liu; Mao‐Bing Fan; Guo‐Qiang Li; Jie Song; Tian‐Ying Xu; Zhi-Yong Li; Yun‐Feng Guan; et al.Pei WangChao‐Yu Miao Hepatic NAD+ deficiency as a therapeutic target for non‐alcoholic fatty liver disease in ageing. British Journal of Pharmacology 2016, 173, 2352-2368, 10.1111/bph.13513.
- Yasir S Elhassan; Katarina Kluckova; Rachel S Fletcher; Mark Schmidt; Antje Garten; Craig L Doig; David M Cartwright; Lucy Oakey; Claire V Burley; Ned Jenkinson; et al.Martin WilsonSamuel J E LucasIldem AkermanAlex SeabrightYu-Chiang LaiDaniel A TennantPeter NightingaleGareth A WallisKonstantinos N ManolopoulosCharles BrennerAndrew PhilpGareth G Lavery Nicotinamide riboside augments the human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures in aged subjects: a placebo-controlled, randomized trial. BioRxiv 2019, n/a, n/a, 10.1101/680462.
- Yasir Elhassan; Katarina Kluckova; Rachel S. Fletcher; Mark Schmidt; Antje Garten; Craig Doig; David M. Cartwright; Lucy Oakey; Claire Burley; Ned Jenkinson; et al.Martin WilsonSamuel LucasIldem AkermanAlex SeabrightYu-Chiang LaiDaniel TennantPeter NightingaleGareth Anthony WallisKonstantinos N. ManolopoulosCharles BrennerAndrew PhilpGareth G. Lavery Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD+ Metabolome and Induces Transcriptomic and Anti-inflammatory Signatures. Cell Reports 2019, 28, 1717-1728.e6, 10.1016/j.celrep.2019.07.043.
- Jade Guest; Ross Grant; Trevor A. Mori; Kevin D. Croft; Changes in Oxidative Damage, Inflammation and [NAD(H)] with Age in Cerebrospinal Fluid. PLOS ONE 2014, 9, e85335, 10.1371/journal.pone.0085335.
- James Clement; Matthew Wong; Anne Poljak; Perminder Sachdev; Nady Braidy; The Plasma NAD+ Metabolome Is Dysregulated in “Normal” Aging. Rejuvenation Research 2019, 22, 121-130, 10.1089/rej.2018.2077.
- Romanas Chaleckis; Itsuo Murakami; Junko Takada; Hiroshi Kondoh; Mitsuhiro Yanagida; Individual variability in human blood metabolites identifies age-related differences. Proceedings of the National Academy of Sciences 2016, 113, 4252-4259, 10.1073/pnas.1603023113.
- Xiao-Hong Zhu; Ming Lu; Byeong-Yeul Lee; Kamil Ugurbil; Wei Chen; In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proceedings of the National Academy of Sciences 2015, 112, 2876-2881, 10.1073/pnas.1417921112.
- Puneet Bagga; Hari Hariharan; Neil E. Wilson; Joanne C. Beer; Russell T. Shinohara; Mark A. Elliott; Joseph A. Baur; Francesco M. Marincola; Walter R. Witschey; Mohammad Haris; et al.John A. DetreRavinder Reddy Single‐Voxel 1 H MR spectroscopy of cerebral nicotinamide adenine dinucleotide (NAD + ) in humans at 7T using a 32‐channel volume coil. Magnetic Resonance in Medicine 2019, 83, 806-814, 10.1002/mrm.27971.
