The term Metabolic Obesity in People with Normal Body Weight (MONW) is used tohas been observed for the first time in 1981 Neil Ruderman, describe people who, despite having a healthy body weight - usually defined by theing a case of patients with symptoms indicative of the metabolic syndromes — reduced insulin sensitivity, hypertension, T2DM, and hypertriglyceridemia — despite normal body mass index (BMI), and more and more often also the percentage of adipose tissue - show. The primary diagnostic criteria were complex and required the use of tests not routinely used in healthy subjects. In later years, the diagnosis was based on the criteria of classic metabolic disorders characteristic of obese peoplesyndrome (MetS). Currently, new criteria are being searched for that will allow for a quick and accurate diagnosis of the MONW.
- MONW
- obesity
- diagnostic criteria
1. The first records of MONW1. Introduction
Metabolically obese normal weight (MONW) was first described in the 1980s, when Ruderman et al.
Modern human lifestyle is not conducive to maintaining health. Sedentary work, low physical activity, improper diet, irregular meals and snacking between them, as well as overeating in the evening, promote obesity[1] described a case of patients with symptoms indicative of the metabolic syndromes—reduced insulin sensitivity, hypertension, T2DM, and hypertriglyceridemia—despite normal body mass index (BMI). In 1989 Ruderman et al.
. According to the definition provided by the World Health Organization (WHO), overweight and obesity are defined as abnormal or excessive fat accumulation that presents a risk to health[2] proposed a scoring system that assessed 22 features (Table 1) that were assigned a specific number of points. Obtaining at least 7 points was equivalent to the diagnosis of MONW.
. Statistics on the percentage of people with excessive adipose tissue are not optimistic. The Global Burden of Disease Group who analyzed data from 68.5 million persons from 195 countries reported in 2017 that between 1980 and 2015, the prevalence of childhood and adult obesity has doubled in 73 countries and shows a steady increase in most other countries [3]. Moreover, the results of Ward et al., suggest that by 2030 every second adult person will have obesity and every fourth adult person will have severe obesity [4].Tabl
2. Biological Mechanisms of MONW
A point scale to identify people with MONW
|
Points |
Symptoms |
||
|
1 |
triglycerides level > 100—150 mg/dL blood presure 125—140/85—90 mmHg weight gain: > 4 after 18 years for women and 21 years for men BMI: 23—25 kg/m2 waist: 71.1—76.2 for women and 86.3—91.4 for men ethnicity: black women, Japanese-Americans, Latinos, |
||
|
2 |
impaired fasting glucose (110—125 mg/dL) triglycerides level > 150 mg/dL blood presure > 140/90 mmHg essential hypertension (under age 60 years) premature coronary heart disease (under age 60 years) low birth weight (< 2.5 kg) inactivity (< 90 min aerobic exercise/week) weight gain: > 8 after 18 years for women and 21 years for men BMI: 25—27 kg/m2 waist: > 76.2 for women and > 91.4 for men uric acid (> 8 mg/dL) |
3 |
gestational diabetes triglycerides level > 150 mg/dL and HDL cholesterol < 35 mg/dL type 2 diabetes mellitus or impaired glucose tolerance hypertriglyceridemia weight gain: > 12 after 18 years for women and 21 years for men premature coronary heart disease (under age 60 years) ethnicity: some American Indian tribes |
|
4 |
type 2 diabetes mellitus impaired glucose tolerance polycystic ovaries |
3. Primary criteria for MONW
This system had its drawbacks, requiring the performance of biochemical tests not routinely performed in healthy people (including uric acid concentration). For this reason, the search for much simpler and more accessible diagnostic criteria was started.
2. A contemporary look at MONW
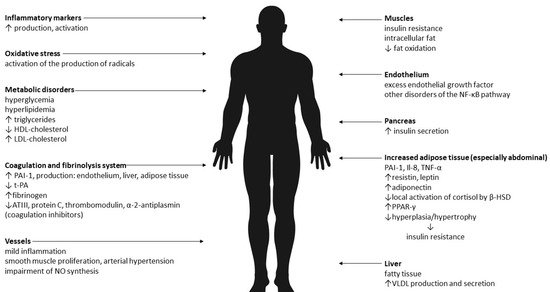
It is now known that in addition to metabolic disorders people with MONW are characterized by an increased content of adipose tissue—in particular, its visceral deposit
The author of the first MONW diagnostic criteria is Ruderman et al.[333]. The assessment of the fat depot is possible after measuring the body composition. This test allows for precise and accurate measurement of individual body components including muscle mass, lean mass and, most importantly, the percentage of adipose tissue (PBF,% BF), the knowledge of which, together with the BMI value, can be used as a screening tool. Among body compositions methods of body composition analysis, dual-energy X-ray absorptiometry (DXA) is considered the “gold standard”.
, who in 1989 proposed a scoring system that assessed 22 features (Table 1) that were assigned a specific number of points. Obtaining at least 7 points was equivalent to the diagnosis of MONW.Currently, the authors of the MONW diagnostics use the developed indicators:
1. the visceral adiposity index (VAI) - which is based on BMI, WC, triglycerides and HDL cholesterol:
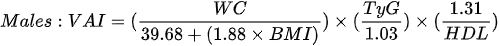
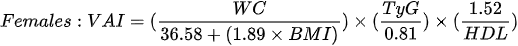
2. the triglycerides–glucose index (TyG) - which is the product of fasting blood glucose and triglycerides:
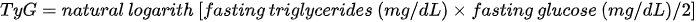
3. lipid accumulation product (LAP) - which is based on the combination of waist circumference measurements and fasting triglycerides:
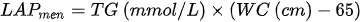
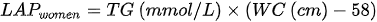
4. the cardiometabolic index (CMI) - which is based on the combination of triglycerides, HDL cholesterol and waist-to-height ratio:
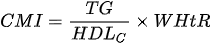
5. metabolic syndrome (MetS) criteria according to the criteria of the National Cholesterol Education Program Adult Treatment Panel III (NCEPATP III) or proposed by the International Diabetes Federation (IDF):
Table 21. Diagnostic criteria for the Metabolic Syndrome.
| Points | Symptoms |
|---|---|
| 1 | triglycerides level > 100–150 mg/dL blood presure 125–140/85–90 mmHg weight gain: >4 after 18 years for women and 21 years for men BMI: 23–25 kg/m2 waist: 71.1–76.2 for women and 86.3–91.4 for men ethnicity: black women, Japanese-Americans, Latinos, Melanesians, Polynesians, New Zealand Maoris |
| 2 | impaired fasting glucose (110–125 mg/dL) triglycerides level > 150 mg/dL blood presure > 140/90 mmHg essential hypertension (under age 60 years) premature coronary heart disease (under age 60 years) low birth weight (<2.5 kg) inactivity (<90 min aerobic exercise/week) weight gain: >8 after 18 years for women and 21 years for men BMI: 25–27 kg/m2 waist: >76.2 for women and >91.4 for men uric acid (>8 mg/dL) ethnicity: Indians, Australian aborigines, Micronesians, Naruans |
| 3 | gestational diabetes triglycerides level > 150 mg/dL and HDL cholesterol < 35 mg/dL type 2 diabetes mellitus or impaired glucose tolerance hypertriglyceridemia weight gain: >12 after 18 years for women and 21 years for men premature coronary heart disease (under age 60 years) ethnicity: some American Indian tribes |
| 4 | type 2 diabetes mellitus impaired glucose tolerance polycystic ovaries |
ethnicity: Indians, Australian aborigines, Micronesians, Naruans |
|
Measure |
NCEPATP III [4] |
IDF [5] |
|
WC |
> 102 cm for men > 88 for women |
≥ 94 cm for men ≥ 80 cm for women * |
|
TG |
> 1.7 mmol/L |
> 1.7 mmol/L or treating hypertriglyceridemia |
|
High-density lipoprotein (HDL) concentration |
< 1.3 mmol/L for men < 1.03 mmol/L for women |
< 1.0 mmol/L for men < 1.3 mmol/L for women or treating said lipid disorder |
|
BP |
> 130/80 mm Hg |
≥ 130 mm Hg systolic or ≥ 85 mm Hg diastolic or treatment of previously diagnosed arterial hypertension; |
|
FG |
> 6.1 mmol/L |
≥ 5.6 mmol/L or drug treatment of |
Legend: WC—waist circumference; TG—concentration of triglycerides; BP—blood pressure; FG—fasting glucose; * in the European population.
3. Conclusions
MONW is undoubtedly a growing problem that should be the focus of further research. Due to the fact that it is a disease that does not show phenotypic signs, screening tests should be carried out, mainly including body composition analysis among young, theoretically healthy people. This will allow for early detection of MONW and appropriate reactions before the occurrence of undesirable consequences—including atherosclerosis or coronary artery disease.
References
- Ruderman, N.B.; Schneider, S.H.; Berchtold, P. The “metabolically-obese,” normal-weight individual. Am. J. Clin. Nutr. 1981, 34, 1617–1621.Kawalec, A.; Kawalec, A. Analysis of the body composition of young adults and the frequency of occurrence of so-called normal weight obesity: A pilot study. Nurs. Public Health 2019, 9, 167–171.
- Ruderman, N.; Chisholm, D.; Pi-Sunyer, X.; Schneider, S. The metabolically obese, normal-weight individual revisited. Diabetes 1998, 47, 699–713.WHO. Obesity: Preventing and Managing the Global Epidemic; WHO: Geneva, Switzerland, 2015.
- Ding, C.; Chan, Z.; Magkos, F. Lean, but not healthy: The “metabolically obese, normal-weight” phenotype. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 408–417.Jones, A.; Tovee, M.; Cutler, L.; Parkinson, K.; Ells, L.; Araujo-Soares, V.; Pearce, M.; Mann, K.; Scott, D.; Harris, J.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. Yearb. Paediatr. Endocrinol. 2018, 15.
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421.Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450.
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645.Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315.
- Dżygadło, B.; Łepecka-Klusek, C.; Pilewski, B. Use of bioelectrical impedance analysis in prevention and treatment of overweight and obesity. Probl. Hig. Epidemiol. 2012, 93, 274–280.
- Bosello, O.; Donataccio, M.P.; Cuzzolaro, M. Obesity or obesities? Controversies on the association between body mass index and premature mortality. Eat. Weight Disord. 2016, 21, 165–174.
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18.
- Cnop, M.; Landchild, M.J.; Vidal, J.; Havel, P.J.; Knowles, N.G.; Carr, D.R.; Wang, F.; Hull, R.L.; Boyko, E.J.; Retzlaff, B.M.; et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: Distinct metabolic effects of two fat compartments. Diabetes 2002, 51, 1005–1015.
- Kokot, I.M.; Pawlik-Sobecka, L.; Płaczkowska, S.; Żółcińska-Wilczyńska, M.; Piwowar, A. The relationship between total body fat and distribution of body fat mass and markers of insulin resistance in young women with normal weight—A pilot study. Clin. Diabetol. 2016, 5, 41–48.
- Wedell-Neergaard, A.-S.; Lang Lehrskov, L.; Christensen, R.H.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Exercise-Induced Changes in Visceral Adipose Tissue Mass Are Regulated by IL-6 Signaling: A Randomized Controlled Trial. Cell Metab. 2019, 29, 844–855.
- Mongraw-Chaffin, M.; Allison, M.A.; Burke, G.L.; Criqui, M.H.; Matsushita, K.; Ouyang, P.; Shah, R.V.; Shay, C.M.; Anderson, C.A.M. CT-derived body fat distribution and incident cardiovascular disease: The multi-ethnic study of atherosclerosis. J. Clin. Endocrinol. Metab. 2017, 102, 4173–4183.
- Ruderman, N.B.; Schneider, S.H.; Berchtold, P. The “metabolically-obese,” normal-weight individual. Am. J. Clin. Nutr. 1981, 34, 1617–1621.
- Katsuki, A.; Sumida, Y.; Urakawa, H.; Gabazza, E.C.; Murashima, S.; Maruyama, N.; Morioka, K.; Nakatani, K.; Yano, Y.; Adachi, Y. Increased Visceral Fat and Serum Levels of Triglyceride Are Associated With Insulin Resistance in Japanese Metabolically Obese, Normal Weight Subjects With Normal Glucose Tolerance. Diabetes Care 2003, 26, 2341–2344.
- Miazgowski, T.; Safranow, K.; Krzyżanowska-Świniarska, B.; Iskierska, K.; Widecka, K. Adiponectin, visfatin and regional fat depots in normal weight obese premenopausal women. Eur. J. Clin. Investig. 2013, 43, 783–790.
- De Lorenzo, A.; Del Gobbo, V.; Premrov, M.G.; Bigioni, M.; Galvano, F.; Di Renzo, L. Normal-weight obese syndrome: Early inflammation? Am. J. Clin. Nutr. 2007, 85, 40–45.
- Katsuki, A.; Sumida, Y.; Urakawa, H.; Gabazza, E.C.; Murashima, S.; Nakatani, K.; Yano, Y.; Adachi, Y. Increased Oxidative Stress Is Associated With Serum Levels of Triglyceride, Insulin Resistance, and Hyperinsulinemia in Japanese Metabolically Obese, Normal-Weight Men. Diabetes Care 2004, 27, 631–632.
- Dvorak, R.V.; DeNino, W.F.; Ades, P.A.; Poehlman, E.T. Phenotypic characteristics associated with insulin resistance in metabolically obese but normal-weight young women. Diabetes 1999, 48, 2210–2214.
- Conus, F.; Allison, D.B.; Rabasa-Lhoret, R.; St-Onge, M.; St-Pierre, D.H.; Tremblay-Lebeau, A.; Poehlman, E.T. Metabolic and behavioral characteristics of metabolically obese but normal-weight women. J. Clin. Endocrinol. Metab. 2004, 89, 5013–5020.
- Stefan, N.; Schick, F.; Häring, H.U. Causes, Characteristics, and Consequences of Metabolically Unhealthy Normal Weight in Humans. Cell Metab. 2017, 26, 292–300.
- Zaid, H.; Antonescu, C.N.; Randhawa, V.K.; Klip, A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem. J. 2008, 413, 201–215.
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252.
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556.
- Poirier, P.; Lemieux, I.; Mauriège, P.; Dewailly, E.; Blanchet, C.; Bergeron, J.; Després, J.P. Impact of waist circumference on the relationship between blood pressure and insulin: The Quebec health survey. Hypertension 2005, 45, 363–367.
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical. Circulation 2006, 113, 898–918.
- Ceriello, A.; Motz, E. Is Oxidative Stress the Pathogenic Mechanism Underlying Insulin Resistance, Diabetes, and Cardiovascular Disease? The Common Soil Hypothesis Revisited. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 816–823.
- Hyun, Y.J.; Koh, S.J.; Chae, J.S.; Kim, J.Y.; Kim, O.Y.; Lim, H.H.; Jang, Y.; Park, S.; Ordovas, J.M.; Lee, J.H. Atherogenecity of LDL and unfavorable adipokine profile in metabolically obese, normal-weight woman. Obesity 2008, 16, 784–789.
- Hajian-Tilaki, K.; Heidari, B. Metabolically healthy obese and unhealthy normal weight in Iranian adult population: Prevalence and the associated factors. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 12, 129–134.
- Lee, K. Metabolically obese but normal weight (MONW) and metabolically healthy but obese (MHO) phenotypes in Koreans: Characteristics and health behaviors. Asia Pac. J. Clin. Nutr 2009, 18, 280–284.
- Wang, B.; Zhuang, R.; Luo, X.; Yin, L.; Pang, C.; Feng, T.; You, H.; Zhai, Y.; Ren, Y.; Zhang, L.; et al. Prevalence of Metabolically Healthy Obese and Metabolically Obese but Normal Weight in Adults Worldwide: A Meta-Analysis. Horm. Metab. Res. 2015, 47, 839–845.
- Li, G.; Li, Y.; Han, L.; Wang, D.; Zhang, Q.; Xiao, X.; Qi, L.; Willi, S.M.; Li, M.; Mi, J.; et al. Interaction between early environment and genetic predisposition instigates the metabolically obese, normal weight phenotype in children: Findings from the BCAMS study. Eur. J. Endocrinol. 2020, 182, 393–403.
- Park, J.M.; Park, D.H.; Song, Y.; Kim, J.O.; Choi, J.E.; Kwon, Y.J.; Kim, S.J.; Lee, J.W.; Hong, K.W. Understanding the genetic architecture of the metabolically unhealthy normal weight and metabolically healthy obese phenotypes in a Korean population. Sci. Rep. 2021, 11, 2279.
- Ruderman, N.; Chisholm, D.; Pi-Sunyer, X.; Schneider, S. The metabolically obese, normal-weight individual revisited. Diabetes 1998, 47, 699–713.
