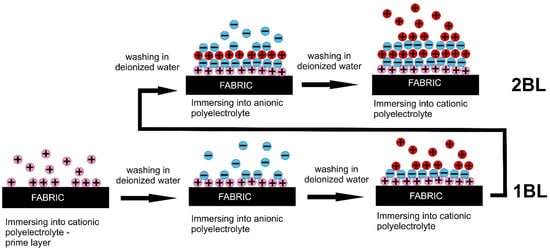
Layer-by-layer (LbL) deposition is an emerging green technology to reduce flammability of the most widely used fibers (cotton, polyester, polyamide and their blends), which shows numerous advantages over current commercially available textile finishing processes due to the use of water as a solvent for a variety of active substances at very low concentrations. The LbL deposition includes immersing textiles into the solutions of oppositely charged polyelectrolytes or spraying textiles with charged solutions to build LbL assemblies with the desired number of bilayers (BLs), trilayers (TLs), or quadlayers (QLs) with different functionality. In conventional LbL deposition, layers are attracted by weak electrostatic forces of polyelectrolytes soluble in water, polyanions and polycations with one charged group per monomer unit, but polymers bearing hydrogen bond donors and acceptors are also able to form assemblies.
- layer-by-layer
- flame retardancy
- cotton
- polyamide
- polyester
- textile
1. Layer-by-Layer Deposition on Textiles
2. Layer-by-Layer Deposition to Reduce Flammability of Cotton
3. Multifunctional Finishing of Cotton
4. Layer-by-Layer Deposition to Reduce Flammability of Polyester
5. Layer-by-Layer Deposition to Reduce Flammability of Polyamide Textiles
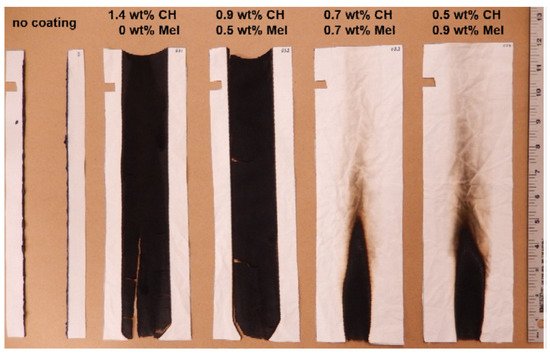
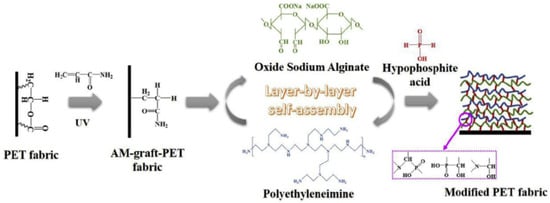
The compounds used for FR LbL deposition of polyamide are similar to those applied to cotton and polyester. According to the literature, polyamide is mainly treated with cationic polymers, such as PAH, CH and PEI, as a primer layer or one of the polyelectrolyte pairs [57][65][66][67][68][125,133,134,135,136]. As a pre-treatment, chemical grafting with PAA as well as enzymatic modification have been reported [69][70][137,138]. Apaydin et al. experimented with a 1 mg/mL cationic PAH solution and a 1 wt% anionic MMT suspension to deposit 5, 10 and 20 BLs on PA6. Cone calorimetry revealed that 20 BLs reduced the pHRR values by more than 60% [65][133]. These same researchers deposited cationic PAH with anionic PSP to build 5, 10, 15 and 40 BLs on PA6.6. TGA showed that the amount of residue increased for 20 and 40 BLs, while the cone calorimeter data showed a significant decrease in pHRR (up to 36%) for all coated fabrics [66][134]. The same group of authors combined cationic PAH, anionic PSP and an anionic suspension of titanium dioxide (TiO2) to deposit 5, 10 and 15 QLs of PAH/PSP/PAH/TiO2 (Section 4.3). Cone calorimetry showed that the coating reduced pHRR by 26% for PA6.6 fabric treated with 15 QLs, but the presence of TiO2 did not significantly improve the FR performance relative to the formulation without TiO2 [57][125]. Kumar Kundu et al. deposited 5, 10 and 15 QLs of cationic CH, anionic PA and anionic oxide sodium alginate (OSA) on PA6.6. The aldehyde groups in OSA formed strong covalent bonds with CH and it could be used in LbL deposition as a cross linker. In the VFT, 10 and 15 QL coatings stopped the melt-dripping of PA6.6, with LOI values of ~22%. Cone calorimetry showed that a maximum reduction (24%) in the pHRR was achieved with five QL deposition [67][135]. In 2018, the same group of authors treated PA6.6 with 5 and 10 BLs of a 1 wt% cationic CH solution and a 2 wt% anionic PA solution to build 5 and 10 BLs. The fabrics were further impregnated in 1 and 5 wt% Na-tetraborate decahydrate solutions and cured at 90 °C. All the treated fabric samples could stop melt dripping in VFTs and pHRR values were lowered compared with the control. In terms of FR performance, the best results were with fabrics treated with 10 BLs (a 31% reduction in pHRR relative to untreated fabric). This coating remained durable up to five washing cycles for PA6.6 impregnated with borate [68][136]. In 2020, they deposited a 1 wt% cationic CH solution and an anionic solution of 1 wt% phosphorylated chitosan (PCH) and 0.25 wt% poly-acrylate sodium (PAS) onto PA6.6 via “one pot” and LbL deposition to compare the efficiency of these two methods in the reduction in the flammability of PA6.6. Fabric treated via “one pot” for 5 and 10 min was then UV-grafted. Layered fabric was immersed first into cationic CH, washed with DI and then immersed into anionic (PCH-PAS), forming 5 and 10 BLs, then either UV-cured (5 and 10 BLs) or thermally crosslinked (10 BLs). The results indicated that the UV-grafted fabric treated with 10 BLs, with a higher weight gain%, exhibited the highest LOI value of 23% and a 25% reduction in pHRR relative to untreated fabric. However, only the thermally cross-linked PA6.6 treated with 10 BLs retained the FR performance after 5 washing cycles [71][139]. One-pot synthesis is an expression denoting that all the reactants are subjected to successive chemical reactions in just one reactor, thus saving time and resources and improving the efficiency of a chemical reaction [72][140]. Ziaur Rahman et al. investigated the influence of pre-treatment and post-treatment on the thermal properties of PA6.6 deposited with two and five BLs of a cationic CH solution with added ME and U and an anionic PA solution. The fabrics were first chemically grafted with PAA in a solution of benzene and dibenzoyl peroxide (BPO). The fabrics were then dipped into cationic PEI, dried at 70 °C and then dipped into a polyacrylic acid–co-maleic acid solution (PAACM) and dried. After LbL treatment, the fabrics were impregnated in a cationic CH and graphene oxide (GO) solution through a pad–dry–cure process. Despite excellent hydrophilic properties achieved by adding GO, none of the treated fabrics passed VFTs either before or after washing [69][137]. In 2020, Jordanov et al. successfully deposited 15–25 BLs of a 1 wt% anionic APP suspension and a 1 wt% cationic CH solution, with added low-molecular-weight compounds 20 wt% THU or U, onto the enzymatically modified surface of PA6.6 fabrics. The process is schematically shown in Figure 67. By adding low-molecular-weight FR compounds into the CH network, the number of BLs passing the HFT was reduced from 25 BLs of APP/CH-U to 15 BLs, while the pHRR was reduced by 35% relative to untreated fabric [70][138]. A summation of polyelectrolytes used to achieve FR of polyamide fabrics is provided in Table 4.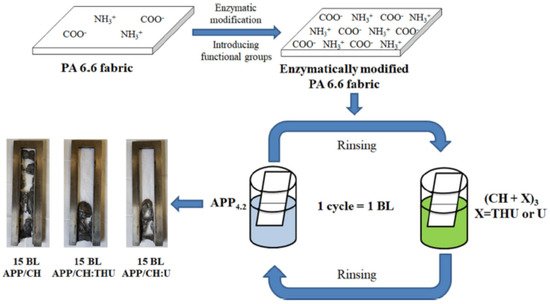
6. Layer-by-Layer Deposition to Reduce Flammability of Cotton/Polyester and Cotton/Polyamide Blends
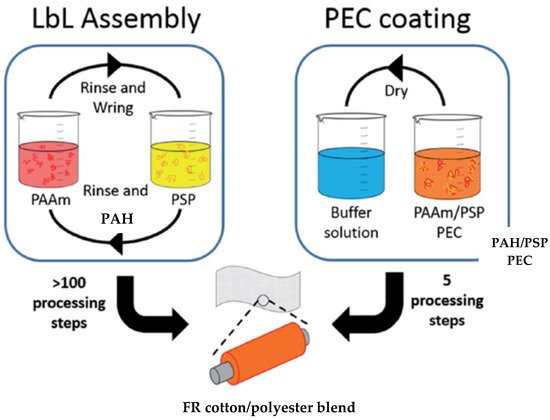
There are very few studies dealing with LbL deposition of cotton/polyester blends to obtain FR properties. Carosio et al. treated cotton/polyester fabrics with a quadlayer (QL) combination of PDAC/PAA/PDAC/APP. The resulting coating limited the flammability of the fabric by suppressing the afterglow and melt dripping, as well as lowering heat release during combustion (Section 4.1) [39][107]. Wattanatanom et al. studied the influence of polyelectrolyte concentration, as well as the number of layers, on the flammability of cotton/polyester blends. By using a cationic BPEI solution and a 5, 7 and 10 wt% anionic APP suspension, the flammability and anti-dripping properties of the fabric were reduced with three, five and seven BLs (Section 4.3). The study showed that the increase in the number of bilayers or the concentration of the solution improved the flame retardancy and anti-dripping of blends by decreasing the after-flame time of coated fabrics and self-extinguishing the flame [59][127]. Alongi et al. investigated whether different orders of layers with the same compounds and same concentration had any influence on the reduction in flammability and anti-dripping behavior of cotton/polyester blends. They used a 0.2 wt% cationic suspension of alumina-coated silica nanoparticles, 0.2 wt% cationic CH solution, 0.2 wt% anionic suspension of silica nanoparticles and 0.2 wt% anionic APP suspension to deposit 5 and 10 silica+/silica-/CH/APP QLs and 5 + 5 and 10 + 10 (CH/APP + silica+/silica−) BLs on fabric blends. The coated fabric did not pass VFTs, proving that only the thickness of the coating and weight gain had an influence on FR properties [73][141]. In 2012, Carosio et al. coated two blend fabrics, one with a 0.2 wt% cationic CH solution and a 0.2 wt% anionic APP suspension, depositing 5, 10 and 20 BLs, and a second fabric with a 0.2 wt% cationic suspension of alumina-coated silica nanoparticles with 0.2 wt% APP. Despite the fact that both FR coatings suppressed the afterglow phenomenon, leaving a remarkable residue after combustion, none of the fabrics passed VFTs [74][142]. In a previous study by Carosio et al. already mentioned in Section 4.1, the burning rate of cotton/polyester blends was successfully reduced in HFTs relative to untreated fabric by combining PDAC/PAA/PDAC/APP in 1, 5 and 10 QLs [107]. In 2016, Haile et al. compared the efficiency of two types of coating, LbL and “one pot” deposition, in extinguishing flames during VFTs, as well as the wash durability to home laundering of FR finishes. Blend fabrics were coated by means of LbL deposition with a 1 wt% cationic PAH solution and a 2 wt% anionic PSP suspension (20, 25 and 30 BLs) and by a “one pot” deposition of a water-soluble polyelectrolyte complex suspension (PEC) consisting of three different wt% concentrations (low, medium and high) of PAH and PSP. The LbL-coated fabric was dried at 70 °C, while “one pot” fabrics were dried and then immersed into a buffer solution consisting of citric acid and sodium citrate at pH 4 for 5 min, as shown in Figure 78. In the acidic environment, PAH and PSP formed an insoluble complex, durable up to five laundry cycles. VFTs showed that the highly concentrated “one pot”-coated cotton/polyester fabric with 17.9% weight gain was able self-extinguish, while the MCC data showed a reduction in pHRR of 78% and 31% for cotton and polyester, respectively. The coating process was reduced from more than 100 processing steps to only 5 [75][143].