Single-cell analysis has become a powerful and indispensable tool in modern biological and medical research. Single-cell isolation is the key step for single-cell analysis. Single-cell printing could utilize various microfluidic technologies for single-cell isolation and analysis, such as droplet microfluidics, microwell arrays, and hydrodynamic traps. Single-cell printing shows several distinct advantages among the single-cell isolation techniques, such as precise deposition, high encapsulation efficiency, and easy recovery. With the development of single-cell printing in the past decade, various single-cell printing-based single-cell analyses and applications have been performed, ranging from single-cell array-based screening and single-cell-based mass spectroscopy to live three-dimensional tissue formation.
- inkjet printing
- cell array
- single-cell analysis
- screening
1. Introduction
As the key step for single-cell analysis, single-cell isolation has attracted great attention, and a number of techniques have been developed for single-cell isolation and manipulation [1][29]. Single-cell printing could utilize various microfluidic technologies for single-cell isolation and analysis, such as droplet microfluidics [2][3][4][6,33,34], microwell arrays [5][6][35,36], and hydrodynamic traps [7][8][37,38]. Single-cell printing has several distinct advantages. First, single-cell printing can effectively and precisely deposit cells at specific sites in high throughput [9][39]. Second, the printed single cells or colonies can be easily recovered with addressability for subsequent analysis. Third, it is convenient to integrate the highly efficient single-cell printing with other techniques, such as imaging system [10][11][40,41], electric field [12][13][14][42,43,44], and acoustic field [15][16][45,46], and the single-cell encapsulation efficiency can reach more than 90%. Furthermore, bioprinting with the single-cell resolution can not only print 2D single-cell arrays for single-cell analysis but also three-dimensional tissue matrixes and organs for tissue engineering, drug discovery, and toxicology [17][18][47,48]. With the development of single-cell printing in the past decade, various single-cell printing-based single-cell analyses and applications have been performed, ranging from single-cell array-based screening [19][20][21][22][23][24][89,90,93,107,108,109] and single-cell-based mass spectroscopy [25][26][27][28][29][30][110,111,112,113,114,115] to live three-dimensional tissue formation [17][18][31][47,48,116].
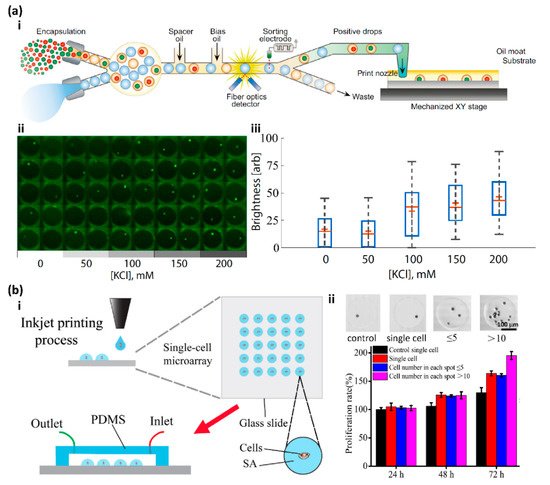
2. High Throughput Screening
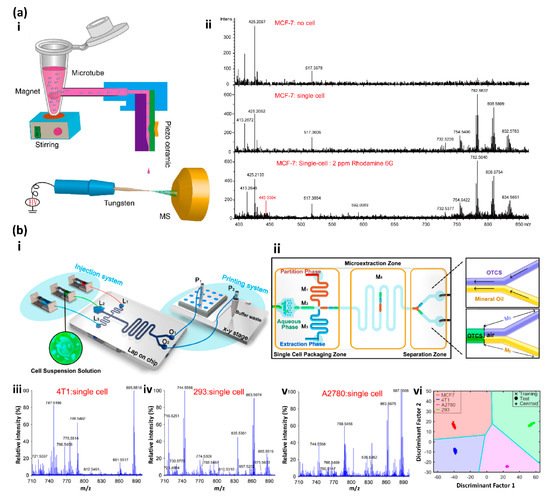
3. Mass Spectrometry-Based Single-Cell Analysis
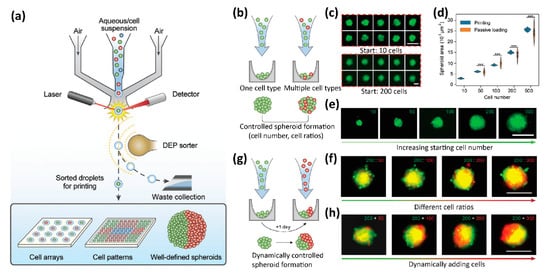
4. 3D Tissue Printing
5. Summary and Future Perspective
Cell bioprinting has made remarkable progress in printing three-dimensional multicellular tissues and organs in the past decade [17][18][39][47,48,74]. However, compared to the 3D bioprinting, where the single-cell resolution is not necessarily required, there is little progress in single-cell printing-based single-cell analysis, although the recently developed single-cell bioprinting strategies show the great potential of single-cell analysis in-depth with promising advantages, such as high encapsulation efficiency, precise deposition, and easy recovery. Currently, the most common strategies for single-cell analysis (omics) are based on droplet microfluidics [3][33]. Several issues limit the applications of current single-cell printing for in-depth single-cell analysis. First and foremost, although the reliability and efficiency of the encapsulation of single cells are dramatically enhanced, the overall throughput of the single-cell printing is still low, at a rate of ~2 Hz [42][99]. Second, compared to the popular microfluidic technologies in an isolated system, current printing strategies normally rely on printing the single-cell droplets in an open environment, which may cause interference and deviation in the subsequent analysis. Third, many subsequent analyses after single-cell isolation are performed on instruments that are incompatible with single-cell printing [43][44][2,7].
Despite the above issues, single-cell printing is experiencing rapid development. Several commercial single-cell printers are now available. Throughput, single-cell efficiency, robustness, and reproducibility are among the most important factors to consider when evaluating a single-cell printing technique or product. With the increasing demand in tissue engineering, precision medicine, liquid biopsy, and drug discovery, we expect that single-cell printing will play a more vital role in single-cell analysis in the future.
