Obesity and insulin resistance are considered as the main underlying risk factors for metabolic disturbances and are involved in the rise of other risk factors, such as hypertension, hyperglycemia, dyslipidemia. The cluster of such risk factors is referred to as metabolic syndrome, a common condition among both human population and animals. Although there are numerous differences between metabolic dysregulation in humans and horses in terms of clinical manifestations, complications, outcomes, etc, a number of disease mechanisms common in both species can be identified (e.g., root causes of metabolic syndrome, role of liver malfunction). The most important pathological factor associated with metabolic syndrome is the affliction of the cardiovascular system in humans and the development of laminitis in horses. The mechanisms that lead to these potentially life-limiting consequences are not fully comparable, although the changes in these species take place in the vascular system. Inflammatory conditions in adipose tissue and effects on metabolic and biochemical processes show similarities between all species.
- Human Metabolic Syndrome
- Equine metabolic syndrome (EMS)
- obesity
1. Human Metabolic Syndrome (MetS)
2. Hyperlipidemias and Hepatic Lipidosis in Horses

2.1. Clinical Signs and Diagnosis
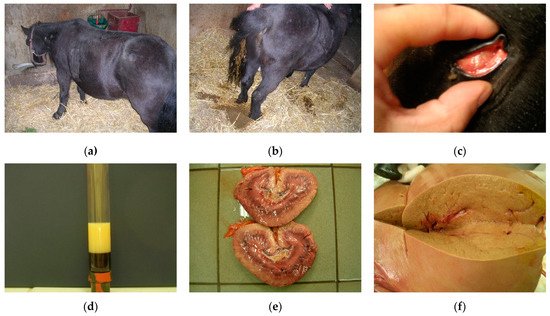
2.2. Prevention and Treatment
3. Comparison between MetS and EMS
References
- Kylin, E. Studien ueber das Hypertonie-Hyperglyka “mie-Hyperurika” miesyndrom. Zent. Inn. Med. 1923, 44, 105–127.
- Vague, J. La differenciation sexuelle-facteur determinant des formes de l’obesite. Presse Med. 1947, 30, 339–340.
- Reaven, G.M. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595.
- Kaplan, N.M. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch. Intern. Med. 1989, 149, 1514–1520.
- Lee, M.-J.; Wu, Y.; Fried, S.K. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol. Asp. Med. 2013, 34, 1–11.
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91.
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711.
- Willerson, J.T.; Ridker, P.M. Inflammation as a Cardiovascular Risk Factor. Circulation 2004, 109, II-2–II-10.
- Cătoi, A.F.; Pârvu, A.; Mureşan, A.; Busetto, L. Metabolic mechanisms in obesity and type 2 diabetes: Insights from bariatric/metabolic surgery. Obes. Facts 2015, 8, 350–363.
- Lorenzatti, A.J.; Toth, P.P. New perspectives on atherogenic dyslipidaemia and cardiovascular disease. Eur. Cardiol. Rev. 2020, 15, 1–9.
- Manchanayake, J.; Chitturi, S.; Nolan, C.; Farrell, G.C. Postprandial hyperinsulinemia is universal in non-diabetic patients with nonalcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2011, 26, 510–516.
- Hsieh, J.; Hayashi, A.A.; Webb, J.; Adeli, K. Postprandial dyslipidemia in insulin resistance: Mechanisms and role of intestinal insulin sensitivity. Atheroscler. Suppl. 2008, 9, 7–13.
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373.
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553.
- Balkau, B.; Charles, M.A. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet. Med. 1999, 16, 442–443.
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480.
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome. Circulation 2009, 120, 1640–1645.
- Zimmet, P.; Alberti, K.G.M.M.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian syndrome: Is the metabolic syndrome and much more! J. Intern. Med. 2019, 286, 181–191.
- N. Frank; Raymond Geor; Simon Bailey; A. E. Durham; P. J. Johnson; Equine Metabolic Syndrome. Journal of Veterinary Internal Medicine 2010, 24, 467-475, 10.1111/j.1939-1676.2010.0503.x.
- Andy E. Durham; Nicholas Frank; Cathy M. McGowan; Nicola J. Menzies‐Gow; Ellen Roelfsema; Ingrid Vervuert; Karsten Feige; Kerstin Fey; ECEIM consensus statement on equine metabolic syndrome. Journal of Veterinary Internal Medicine 2018, 33, 335-349, 10.1111/jvim.15423.
- McKenzie, H.C., III. Equine hyperlipidemias. Vet. Clin. N. Am. Equine Pract. 2011, 27, 59–72.
- Dunkel, B.; McKenzie, H.C., III. Severe hypertriglyceridaemia in clinically ill horses: Diagnosis, treatment and outcome. Equine Vet. J. 2003, 35, 590–595.
- Naylor, J.M.; Kronfeld, D.S.; Acland, H. Hyperlipemia in horses: Effects of undernutrition and Disease. Am. J. Vet. Res. 1980, 41, 899–905.
- Field, J. Hyperlipaemia in a Quarterhorse. Comp. Cont. Educ. Pract. 1987, 10, 218–221.
- McAuliffe, S. Knottenbelt and Pascoe’s Color Atlas of Diseases and Disorders of the Horse E-Book, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014.
- Mogg, T.D.; Palmer, J.E. Hyperlipidemia, hyperlipemia, and hepatic lipidosis in American miniature horses: 23 cases (1990–1994). J. Am. Vet. Med. Assoc. 1995, 207, 604–607.
- Moore, B.R.; Abood, S.K.; Hinchcliff, K.W. Hyperlipemia in 9 miniature horses and miniature donkeys. J. Vet. Intern. Med. 1994, 8, 376–381.
- Reid, S.W.; Mohammed, H.O. Survival analysis approach to risk factors associated with hyperlipemia in donkeys. J. Am. Vet. Med. Assoc. 1996, 209, 1449–1452.
- Jeffcott, L.B.; Field, J.R.; McLean, J.G.; O’Dea, K. Glucose tolerance and insulin sensitivity in ponies and Standardbred horses. Equine Vet. J. 1986, 18, 97–101.
- Watson, T.D.; Murphy, D.; Love, S. Equine hyperlipaemia in the United Kingdom: Clinical features and blood biochemistry of 18 cases. Vet. Rec. 1992, 131, 48–51.
- Ralston, S. Hyperglycemia/hyperinsulinemia after feeding a meal of grain to young horses with osteochondritis Dissecans (OCD) lesions. Pferdeheilkunde Equine Med. 1996, 12, 320–322.
- Foreman, J.H. Hyperlipemia and Hepatic Lipidosis in Large Animals. Available online: https://www.msdvetmanual.com/Dig.estive-system/hepatic-Disease-in-large-animals/hyperlipemia-and-hepatic-lipidosis-in-large-animals#v3265716 (accessed on 12 September 2021).
- Watson, T.D.; Burns, L.; Packard, C.J.; Shepherd, J. Effects of pregnancy and lactation on plasma lipid and lipoprotein concentrations, lipoprotein composition and post-heparin lipase activities in Shetland pony mares. J. Reprod Fertil. 1993, 97, 563–568.
- Gilbert, R.O. Congenital hyperlipaemia in a Shetland pony foal. Equine Vet. J. 1986, 18, 498–500.
- Gan, S.I.; Edwards, A.L.; Symonds, C.J.; Beck, P.L. Hypertriglyceridemia-induced pancreatitis: A case-based review. World J. Gastroenterol. 2006, 12, 7197–7202.
- Breidenbach, A.; Fuhrmann, H.; Deegen, E.; Lindholm, A.; Sallmann, H.P. Studies on equine lipid Metabolism. Lipolytic activities of plasma and tissue lipases in large horses and ponies. J. Vet. Med. Ser. A 1999, 46, 39–48.
- Van Weyenberg, S.; Hesta, M.; Buyse, J.; Janssens, G.P. The effect of weight loss by energy restriction on Metabolic profile and glucose tolerance in ponies. J. Anim. Physiol. Anim. Nutr. 2008, 92, 538–545.
- Geor, R.J.; Harris, P. Dietary management of obesity and insulin resistance: Countering risk for laminitis. Vet. Clin. N. Am. Equine Pract. 2009, 25, 51–65.
- Hughes, K.J.; Hodgson, D.R.; Dart, A.J. Equine hyperlipaemia: A review. Aust. Vet. J. 2004, 82, 136–142.
- Schmidt, O.; Deegen, E.; Fuhrmann, H.; Dühlmeier, R.; Sallmann, H.P. Effects of fat feeding and energy level on plasma Metabolites and hormones in Shetland ponies. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2001, 48, 39–49.
- Bamford, N.J.; Potter, S.J.; Baskerville, C.L.; Harris, P.A.; Bailey, S.R. Effect of increased adiposity on insulin sensitivity and adipokine concentrations in different equine breeds adapted to cereal-rich or fat-rich meals. Vet. J. 2016, 214, 14–20.
- Pratt, S.E.; Geor, R.J.; McCutcheon, L.J. Effects of dietary energy source and physical conditioning on insulin sensitivity and glucose tolerance in Standardbred horses. Equine Vet. J. 2006, 38, 579–584.
- Quinn, R.W.; Burk, A.O.; Hartsock, T.G.; Petersen, E.D.; Whitley, N.C.; Treiber, K.H.; Boston, R.C. Insulin sensitivity in Thoroughbred geldings: Effect of weight gain, diet, and exercise on insulin sensitivity in Thoroughbred geldings. J. Equine Vet. Sci. 2008, 28, 728–738.
- Jacob, S.I.; Geor, R.J.; Weber, P.S.D.; Harris, P.A.; McCue, M.E. Effect of age and dietary carbohydrate profiles on glucose and insulin dynamics in horses. Equine Vet. J. 2017, 50, 249–254.
- Chameroy, K.A.; Frank, N.; Elliott, S.B.; Boston, R.C. Comparison of plasma active glucagon-like peptide 1 concentrations in normal horses and those with equine Metabolic syndrome and in horses placed on a high-grain diet. J. Equine Vet. Sci. 2016, 40, 16–25.
- Krause, J.B.; McKenzie, H.C., III. Parenteral nutrition in foals: A retrospective study of 45 cases (2000–2004). Equine Vet. J. 2007, 39, 74–78.
- Magdesian, K.G. Parenteral nutrition in the mature horse. Equine Vet. Educ. 2010, 22, 364–371.
- Durham, A.E.; Rendle, D.I.; Newton, J.E. The effect of metformin on measurements of insulin sensitivity and beta cell response in 18 horses and ponies with insulin resistance. Equine Vet. J. 2008, 40, 493–500.
- Frank, N.; Sommardahl, C.S.; Eiler, H.; Webb, L.L.; Denhart, J.W.; Boston, R.C. Effects of oral administration of levothyroxine sodium on concentrations of plasma lipids, concentration and composition of very-low-density lipoproteins, and glucose dynamics in healthy adult mares. Am. J. Vet. Res. 2005, 66, 1032–1038.
- Rendle, D.I.; Rutledge, F.; Hughes, K.J.; Heller, J.; Durham, A.E. Effects of metformin hydrochloride on blood glucose and insulin responses to oral dextrose in horses. Equine Vet. J. 2013, 45, 751–754.
- Tinworth, K.D.; Boston, R.C.; Harris, P.A.; Sillence, M.N.; Raidal, S.L.; Noble, G.K. The effect of oral metformin on insulin sensitivity in insulin-resistant ponies. Vet. J. 2012, 191, 79–84.
- Frank, N. Equine Metabolic syndrome. Vet. Clin. N. Am. Equine Pract. 2011, 27, 73–92.
- Waitt, L.H.; Cebra, C.K. Characterization of hypertriglyceridemia and response to treatment with insulin in horses, ponies, and donkeys: 44 cases (1995–2005). J. Am. Vet. Med. Assoc. 2009, 234, 915–919.
- Cole, R.P. Heparin treatment for aevere hypertriglyceridemia in diabetic ketoacidosis. Arch. Intern. Med. 2009, 169, 1439–1441.
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97.
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004, 25, 4–7.
- Vick, M.M.; Adams, A.A.; Murphy, B.A.; Sessions, D.R.; Horohov, D.W.; Cook, R.F.; Shelton, B.J.; Fitzgerald, B.P. Relationships among inflammatory cytokines, obesity, and insulin sensitivity in the horse. J. Anim. Sci. 2007, 85, 1144–1155.
- Vick, M.M.; Murphy, B.A.; Sessions, D.R.; Reedy, S.E.; Kennedy, E.L.; Horohov, D.W.; Cook, R.F.; Fitzgerald, B.P. Effects of systemic inflammation on insulin sensitivity in horses and inflammatory cytokine expression in adipose tissue. Am. J. Vet. Res. 2008, 69, 130–139.
- Yudkin, J.S.; Stehouwer, C.D.; Emeis, J.J.; Coppack, S.W. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler. Thromb. Vasc. Biol. 1999, 19, 972–978.
- Treiber, K.; Carter, R.; Gay, L.; Williams, C.; Geor, R. Inflammatory and redox status of ponies with a history of pasture-associated laminitis. Vet. Immunol. Immunopathol. 2009, 129, 216–220.
- de Laat, M.A.; Clement, C.K.; McGowan, C.M.; Sillence, M.N.; Pollitt, C.C.; Lacombe, V.A. Toll-like receptor and pro-inflammatory cytokine expression during prolonged hyperinsulinaemia in horses: Implications for laminitis. Vet. Immunol. Immunopathol. 2014, 157, 78–86.
- Corcoran, M.P.; Lamon-Fava, S.; Fielding, R.A. Skeletal muscle lipid deposition and insulin resistance: Effect of dietary fatty acids and exercise. Am. J. Clin. Nutr. 2007, 85, 662–677.
- Eckardt, K.; Taube, A.; Eckel, J. Obesity-associated insulin resistance in skeletal muscle: Role of lipid accumulation and physical inactivity. Rev. Endocr. Metab. Disord. 2011, 12, 163–172.
- Einstein, F.H.; Huffman, D.M.; Fishman, S.; Jerschow, E.; Heo, H.J.; Atzmon, G.; Schechter, C.; Barzilai, N.; Muzumdar, R.H. Aging per se Increases the Susceptibility to Free Fatty Acid–Induced Insulin Resistance. J. Gerontol. Ser. A 2010, 65A, 800–808.
- Treiber, K.H.; Kronfeld, D.S.; Hess, T.M.; Byrd, B.M.; Splan, R.K.; Staniar, W.B. Evaluation of genetic and Metabolic predispositions and nutritional risk factors for pasture-associated laminitis in ponies. J. Am. Vet. Med. Assoc. 2006, 228, 1538–1545.
- Geelen, S.N.; Jansen, W.L.; Sloet van Oldruitenborgh-Oosterbaan, M.M.; Breukink, H.J.; Beynen, A.C. Fat feeding increases equine heparin-released lipoprotein lipase activity. J. Vet. Intern. Med. 2001, 15, 478–481.
- Frank, N.; Elliott, S.B.; Brandt, L.E.; Keisler, D.H. Physical characteristics, blood hormone concentrations, and plasma lipid concentrations in obese horses with insulin resistance. J. Am. Vet. Med. Assoc. 2006, 228, 1383–1390.
- Carr, M.C.; Brunzell, J.D. Abdominal obesity and dyslipidemia in the Metabolic syndrome: Importance of type 2 diabetes and familial combined hyperlipidemia in coronary artery Disease risk. J. Clin. Endocrinol. Metab. 2004, 89, 2601–2607.
- Watson, T.D.; Packard, C.J.; Shepherd, J. Plasma lipid transport in the horse (Equus caballus). Comp. Biochem. Physiol. B Comp. Biochem. 1993, 106, 27–34.
- McCue, M.E.; Geor, R.J.; Schultz, N. Equine Metabolic syndrome: A complex Disease influenced by genetics and the environment. J. Equine Vet. Sci. 2015, 35, 367–375.
- Perreault, M.; Zulyniak, M.A.; Badoud, F.; Stephenson, S.; Badawi, A.; Buchholz, A.; Mutch, D.M. A Distinct fatty acid profile underlies the reduced inflammatory state of Metabolically healthy obese individuals. PLoS ONE 2014, 9, e88539.
- Calori, G.; Lattuada, G.; Piemonti, L.; Garancini, M.P.; Ragogna, F.; Villa, M.; Mannino, S.; Crosignani, P.; Bosi, E.; Luzi, L.; et al. Prevalence, Metabolic features, and prognosis of Metabolically healthy obese Italian individuals: The Cremona study. Diabetes Care 2010, 34, 210–215.
- Phillips, C.M. Metabolically healthy obesity: Definitions, determinants and clinical implications. Rev. Endocr. Metab. Disord. 2013, 14, 219–227.
- Engelsen, C.D.; Gorter, K.J.; Salomé, P.L.; Rutten, G.E. Development of Metabolic syndrome components in adults with a healthy obese phenotype: A 3-year follow-up. Obesity 2013, 21, 1025–1030.
- Schlaich, M.; Straznicky, N.; Lambert, E.; Lambert, G. Metabolic syndrome: A sympathetic Disease? Lancet Diabetes Endocrinol. 2015, 3, 148–157.
- Carter, R.A.; Geor, R.J.; Burton Staniar, W.; Cubitt, T.A.; Harris, P.A. Apparent adiposity assessed by standardised scoring systems and morphometric measurements in horses and ponies. Vet. J. 2009, 179, 204–210.
- Carter, R.A.; Treiber, K.H.; Geor, R.J.; Douglass, L.; Harris, P.A. Prediction of incipient pasture-associated laminitis from hyperinsulinaemia, hyperleptinaemia and generalised and localised obesity in a cohort of ponies. Equine Vet. J. 2009, 41, 171–178.
- Noor, S.; Zubair, M.; Ahmad, J. Diabetic foot ulcer—A review on pathophysiology, classification and microbial etiology. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 192–199.
- Papanas, N.; Ziegler, D. Risk factors and comorbidities in diabetic neuropathy: An update. Rev. Diabet. Stud. 2015, 12, 48–62.
- Jones, E.; Viñuela-Fernandez, I.; Eager, R.A.; Delaney, A.; Anderson, H.; Patel, A.; Robertson, D.C.; Allchorne, A.; Sirinathsinghji, E.C.; Milne, E.M.; et al. Neuropathic changes in equine laminitis pain. Pain 2007, 132, 321–331.
- Zamboulis, D.E.; Senior, M.; Clegg, P.D.; Milner, P.I. Expression of purinergic P2X receptor subtypes 1, 2, 3 and 7 in equine laminitis. Vet. J. 2013, 198, 472–478.
- Driessen, B.; Bauquier, S.H.; Zarucco, L. Neuropathic pain management in chronic laminitis. Vet. Clin. N. Am. Equine Pract. 2010, 26, 315–337.
- Chang-Chen, K.J.; Mullur, R.; Bernal-Mizrachi, E. Beta-cell failure as a complication of diabetes. Rev. Endocr. Metab. Disord. 2008, 9, 329–343.
- Rajaie, S.; Azadbakht, L.; Khazaei, M.; Sherbafchi, M.; Esmaillzadeh, A. Moderate replacement of carbohydrates by dietary fats affects features of Metabolic syndrome: A randomized crossover clinical trial. Nutrition 2014, 30, 61–68.
- Imamura, F.; Micha, R.; Wu, J.H.Y.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: A systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016, 13, e1002087.
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2013, 17, 1689–1696.
- Ragno, V.M.; Zello, G.A.; Klein, C.D.; Montgomery, J.B. From Table to Stable: A Comparative Review of Selected Aspects of Human and Equine Metabolic Syndrome. J. Equine Vet. Sci. 2019, 79, 131–138.
- Carr, E.A. Enteral/Parenteral Nutrition in Foals and Adult Horses Practical Guidelines for the Practitioner. Vet. Clin. N. Am. Equine Pract. 2018, 34, 169–180.
- Golenz, M.R.; Knight, D.A.; Yvorchuk-St Jean, K.E. Use of a human enteral feeding preparation for treatment of hyperlipemia and nutritional support during healing of an esophageal laceration in a miniature horse. J. Am. Vet. Med. Assoc. 1992, 200, 951–953.
- Magdesian, K.G. Nutrition for critical gastrointestinal illness: Feeding horses with diarrhea or colic. Vet. Clin. N. Am. Equine Pract. 2003, 19, 617–644.
- Lewis, S.J.; Andersen, H.K.; Thomas, S. Early Enteral Nutrition Within 24 h of Intestinal Surgery Versus Later Commencement of Feeding: A Systematic review and Meta-analysis. J. Gastrointest. Surg. 2008, 13, 569.
- Borghouts, L.B.; Keizer, H.A. Exercise and insulin sensitivity: A review. Int. J. Sports Med. 2000, 21, 1–12.
- Durham, A.E.; Hughes, K.J.; Cottle, H.J.; Rendle, D.I.; Boston, R.C. Type 2 diabetes mellitus with pancreatic β cell dysfunction in 3 horses confirmed with minimal model analysis. Equine Vet. J. 2009, 41, 924–929.
- Fletcher, B.; Gulanick, M.; Lamendola, C. Risk factors for type 2 diabetes mellitus. J. Cardiovasc. Nurs. 2002, 16, 17–23.
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J. Med. Sci. 2014, 11, 1185–1200.
- Durham, A.E. Endocrine Disease in Aged Horses. Vet. Clin. N. Am. Equine Pract. 2016, 32, 301–315.
- Durham, A.E. Therapeutics for Equine Endocrine Disorders. Vet. Clin. N. Am. Equine Pract. 2017, 33, 127–139.
