In the late 1930s and early 1940s, it was discovered that the substitution on aromatic rings of hydrogen atoms with chlorine yielded a novel chemistry of antimicrobials. However, within a few years, many of these compounds and formulations showed adverse effects, including human toxicity, ecotoxicity, and unwanted environmental persistence and bioaccumulation, quickly leading to regulatory bans and phase-outs. Among these, the triclocarban, a polychlorinated aromatic antimicrobial agent, was employed as a major ingredient of toys, clothing, food packaging materials, food industry floors, medical supplies, and especially of personal care products, such as soaps, toothpaste, and shampoo. Triclocarban has been widely used for over 50 years, but only recently some concerns were raised about its endocrine disruptive properties. In September 2016, the U.S. Food and Drug Administration banned its use in over-the-counter hand and body washes because of its toxicity. The withdrawal of triclocarban has prompted the efforts to search for new antimicrobial compounds and several analogues of triclocarban have also been studied. In this review, an examination of different facets of triclocarban and its analogues will be analyzed.
- antimicrobials
- diarylureas
- bis-arylureas
- triclocarban
1. Introduction
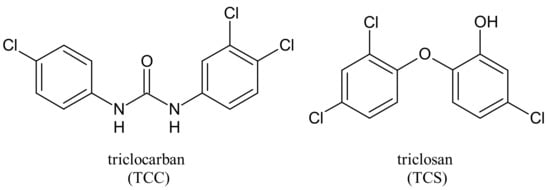
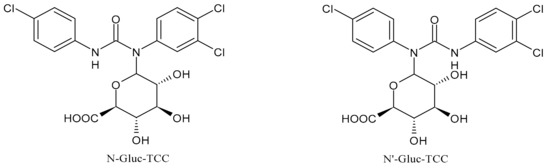
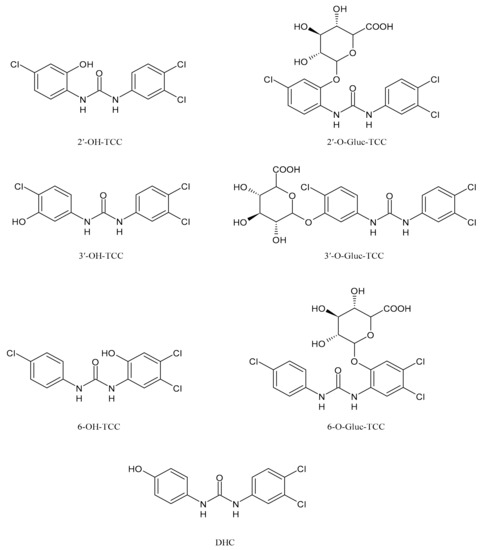
2. Metabolism and Transformation Products of TCC
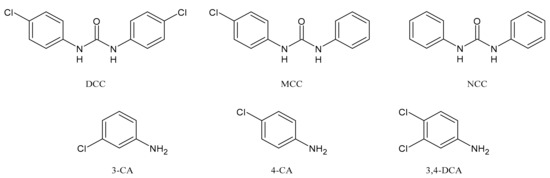
3. Biological Activity of TCC
References
- Liao, C.; Kannan, K. A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Arch. Environ. Contam. Toxicol. 2014, 67, 50–59.
- Asimakopoulos, A.G.; Xue, J.; De Carvalho, B.P.; Iyer, A.; Abualnaja, K.O.; Yaghmoor, S.S.; Kumosani, T.A.; Kannan, K. Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in Jeddah, Saudi Arabia. Environ. Res. 2016, 150, 573–581.
- Xie, X.; Lu, C.; Wu, M.; Liang, J.; Ying, Y.; Liu, K.; Huang, X.; Zheng, S.; Du, X.; Liu, D.; et al. Association between Triclocarban and Triclosan Exposures and the Risks of Type 2 Diabetes Mellitus and Impaired Glucose Tolerance in the National Health and Nutrition Examination Survey (NHANES 2013–2014). Environ. Int. 2020, 136, 105445.
- Tran, T.M.; Trinh, H.T.; Anh, H.Q.; Van Le, T.; Le, S.N.; Minh, T.B. Characterization of triclosan and triclocarban in indoor dust from home micro-environments in Vietnam and relevance of non-dietary exposure. Sci. Total Environ. 2020, 732, 139326.
- Gomes, M.F.; de Paula, V.D.C.S.; Martins, L.R.R.; Garcia, J.R.E.; Yamamoto, F.Y.; de Freitas, A.M. Sublethal effects of triclosan and triclocarban at environmental concentrations in silver catfish (Rhamdia quelen) embryos. Chemosphere 2021, 263, 127985.
- Catalano, A.; Iacopetta, D.; Sinicropi, M.S.; Franchini, C. Diarylureas as Antitumor Agents. Appl. Sci. 2021, 11, 374.
- Qiao, L.; Hao, S. Novel trifluoromethylcoumarinyl urea derivatives: Synthesis, characterization, fluorescence, and bioactivity. Molecules 2018, 23, 600.
- Xue, D.; Chen, W.; Neamati, N. Discovery, structure-activity relationship study and biological evaluation of 2-thioureidothiophene-3-carboxylates as a novel class of CXC chemokine receptor 2 (CXCR2) antagonists. Eur. J. Med. Chem. 2020, 204, 112387.
- Aghcheli, A.; Toolabi, M.; Ayati, A.; Moghimi, S.; Firoozpour, L.; Bakhshaiesh, T.O.; Nazeri, E.; Norouzbahari, M.; Esmaeili, R.; Foroumadi, A. Design, synthesis, and biological evaluation of 1-(5-(benzylthio)-1, 3, 4-thiadiazol-2-yl)-3-phenylurea derivatives as anticancer agents. Med. Chem. Res. 2020, 29, 2000–2010.
- Perrey, D.A.; Zhang, Y. Therapeutics development for addiction: Orexin-1 receptor antagonists. Brain Res. 2020, 1731, 145922.
- Podlewska, S.; Bugno, R.; Lacivita, E.; Leopoldo, M.; Bojarski, A.J.; Handzlik, J. Low basicity as a characteristic for atypical ligands of serotonin receptor 5-HT2. Int. J. Mol. Sci. 2021, 22, 1035.
- Catalano, A. COVID-19: Could Irisin Become the Handyman Myokine of the 21st Century? Coronaviruses 2020, 1, 32–41.
- Catalano, A.; Iacopetta, D.; Pellegrino, M.; Aquaro, S.; Franchini, C.; Sinicropi, M.S. Diarylureas: Repositioning from antitumor to antimicrobials or multi-target agents against new pandemics. Antibiotics 2021, 10, 92.
- Sreevidya, V.S.; Lenz, K.A.; Svoboda, K.R.; Ma, H. Benzalkonium chloride, benzethonium chloride, and chloroxylenol—Three replacement antimicrobials are more toxic than triclosan and triclocarban in two model organisms. Environ. Pollut. 2018, 235, 814–824.
- Arifin, S.N.H.; Mohamed, R.; Al-Gheethi, A.; Lai, C.W.; Yashni, G. Heterogeneous photocatalysis of triclocarban and triclosan in greywater: A systematic and bibliometric review analysis. Int. J. Environ. Anal. Chem. 2021, 1–19.
- Asimakopoulos, A.G.; Elangovan, M.; Kannan, K. Migration of parabens, bisphenols, benzophenone-type UV filters, triclosan, and triclocarban from teethers and its implications for infant exposure. Environ. Sci. Technol. 2016, 50, 13539–13547.
- Moreta, C.; Tena, M.T.; Kannan, K. Analytical method for the determination and a survey of parabens and its derivatives in pharmaceuticals. Environ. Res. 2015, 142, 452–460.
- Li, W.; Zhang, W.; Chang, M.; Ren, J.; Xie, W.; Chen, H.; Zhang, Z.; Zhuang, X.; Shen, G.; Li, H. Metabonomics reveals that triclocarban affects liver metabolism by affecting glucose metabolism, β-oxidation of fatty acids, and the TCA cycle in male mice. Toxicol. Lett. 2018, 299, 76–85.
- European Commission, Scientific Committee on Consumer Products. Opinion on Triclocarban for Other Uses than as a Preservative; Colipa no. P29. SCCP/0851/04; European Commission: Brussels, Belgium, 2005.
- Musee, N. Environmental risk assessment of triclosan and triclocarban from personal care products in South Africa. Environ. Pollut. 2018, 242, 827–838.
- Chaudhari, U.; Nemade, H.; Sureshkumar, P.; Vinken, M.; Ates, G.; Rogiers, V.; Hescheler, J.; Hengstler, J.G.; Sachinidis, A. Functional cardiotoxicity assessment of cosmetic compounds using human-induced pluripotent stem cell-derived cardiomyocytes. Arch. Toxicol. 2018, 92, 371–381.
- Halden, R.U.; Paul, D.H. Co-occurence of triclocarban and triclosan in U.S. water resources. Environ. Sci. Technol. 2005, 39, 1420–1426.
- Yang, H.; Sanidad, K.Z.; Wang, W.; Xie, M.; Gu, M.; Cao, X.; Xiao, H.; Zhang, G. Triclocarban exposure exaggerates colitis and colon tumorigenesis: Roles of gut microbiota involved. Gut Microbes 2020, 12, 1690364.
- Ye, X.; Wong, L.Y.; Dwivedi, P.; Zhou, X.; Jia, T.; Calafat, A.M. Urinary concentrations of the antibacterial agent triclocarban in United States residents: 2013−2014 National health and nutrition examination survey. Environ. Sci. Technol. 2016, 50, 13548–13554.
- Halden, R.U.; Lindeman, A.E.; Aiello, A.E.; Andrews, D.; Arnold, W.A.; Fair, P.; Fuoco, R.E.; Geer, L.A.; Johnson, P.I.; Lohmann, R.; et al. The Florence statement on triclosan and triclocarban. Environ. Health Perspect. 2017, 125, 064501.
- CDC (Centers for Disease Control and Prevention). Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). 52(RR10). 2003. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5210a1.htm (accessed on 15 April 2021).
- Guedes-Alonso, R.; Montesdeoca-Esponda, S.; Herrera-Melián, J.A.; Rodríguez-Rodríguez, R.; Ojeda-González, Z.; Landívar-Andrade, V.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Pharmaceutical and personal care product residues in a macrophyte pond-constructed wetland treating wastewater from a university campus: Presence, removal and ecological risk assessment. Sci. Total Environ. 2020, 703, 135596.
- Ahn, K.C.; Zhao, B.; Chen, J.; Cherednichenko, G.; Sanmarti, E.; Denison, M.S.; Lasley, B.; Pessah, I.N.; Kultz, D.; Chang, D.P.; et al. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: Receptor-based bioassay screens. Environ. Health Perspect. 2008, 116, 1203–1210.
- Tarnow, P.; Tralau, T.; Hunecke, D.; Luch, A. Effects of triclocarban on the transcription of estrogen, androgen and aryl hydrocarbon receptor responsive genes in human breast cancer cells. Toxicol. Vitro 2013, 27, 1467–1475.
- Vimalkumar, K.; Arun, E.; Krishna-Kumar, S.; Poopal, R.K.; Nikhil, N.P.; Subramanian, A.; Ramaswamy, B.R. Occurrence of triclocarban and benzotriazole ultraviolet stabilizers in water, sediment, and fish from Indian rivers. Sci. Total Environ. 2018, 625, 1351–1360.
- Subedi, B.; Lee, S.; Moon, H.B.; Kannan, K. Emission of artificial sweeteners, select pharmaceuticals, and personal care products through sewage sludge from wastewater treatment plants in Korea. Environ. Int. 2014, 68, 33–40.
- Karthikraj, R.; Kannan, K.; Karthikraj, R. Mass loading and removal of benzotriazoles, benzothiazoles, benzophenones, and bisphenols in Indian sewage treatment plants. Chemosphere 2017, 181, 216–223.
- Vimalkumar, K.; Seethappan, S.; Pugazhendhi, A. Fate of Triclocarban (TCC) in aquatic and terrestrial systems and human exposure. Chemosphere 2019, 230, 201–209.
- Abbott, T.; Kor-Bicakci, G.; Islam, M.S.; Eskicioglu, C. A review on the fate of legacy and alternative antimicrobials and their metabolites during wastewater and sludge treatment. Int. J. Mol. Sci. 2020, 21, 9241.
- Xia, K.; Bhandari, A.; Das, K.; Pillar, G. Occurrence and Fate of Pharmaceuticals and Personal Care Products (PPCPs) in Biosolids. J. Environ. Qual. 2005, 34, 91–104.
- Onesios, K.M.; Yu, J.T.; Bouwer, E.J. Biodegradation and removal of pharmaceuticals and personal care products in treatment systems: A review. Biodegradation 2009, 20, 441–466.
- Thelusmond, J.R.; Kawka, E.; Strathmann, T.J.; Cupples, A.M. Diclofenac, carbamazepine and triclocarban biodegradation in agricultural soils and the microorganisms and metabolic pathways affected. Sci. Total Environ. 2018, 640, 1393–1410.
- Armstrong, D.L.; Rice, C.P.; Ramirez, M.; Torrents, A. Influence of thermal hydrolysis-anaerobic digestion treatment of wastewater solids on concentrations of triclosan, triclocarban, and their transformation products in biosolids. Chemosphere 2017, 171, 609–616.
- Yun, H.; Liang, B.; Kong, D.; Li, X.; Wang, A. Fate, risk and removal of triclocarban: A critical review. J. Hazard. Mater. 2020, 387, 121944.
- Yin, J.; Wei, L.; Shi, Y.; Zhang, J.; Wu, Q.; Shao, B. Chinese population exposure to triclosan and triclocarban as measured via human urine and nails. Environ. Geochem. Health 2016, 38, 1125–1135.
- Wei, L.; Qiao, P.; Shi, Y.; Ruan, Y.; Yin, J.; Wu, Q.; Shao, B. Triclosan/triclocarban levels in maternal and umbilical blood samples and their association with fetal malformation. Clin. Chim. Acta 2017, 466, 133–137.
- Buck Louis, G.M.; Smarr, M.M.; Sun, L.; Chen, Z.; Honda, M.; Wang, W.; Karthikraj, R.; Weck, J.; Kannan, K. Endocrine disrupting chemicals in seminal plasma and couple fecundity. Environ. Res. 2018, 163, 64–70.
- Li, A.J.; Xue, J.; Lin, S.; Al-Malki, A.L.; Al-Ghamdi, M.A.; Kumosani, T.A.; Kannan, K. Urinary Concentrations of Environmental Phenols and Their Association with Type 2 Diabetes in a Population in Jeddah, Saudi Arabia. Environ. Res. 2018, 166, 544–552.
- Rocha, B.A.; Asimakopoulos, A.G.; Honda, M.; da Costa, N.L.; Barbosa, R.M.; Barbosa, F., Jr.; Kannan, K. Advanced data mining approaches in the assessment of urinary concentrations of bisphenols, chlorophenols, parabens and benzophenones in Brazilian children and their association to DNA damage. Environ. Int. 2018, 116, 269–277.
- Iyer, A.P.; Xue, J.; Honda, M.; Robinson, M.; Kumosani, T.A.; Abulnaja, K.; Kannan, K. Urinary levels of triclosan and triclocarban in several Asian countries, Greece and the USA: Association with oxidative stress. Environ. Res. 2018, 160, 91–96.
- Brose, D.A.; Kumar, K.; Liao, A.; Hundal, L.S.; Tian, G.; Cox, A.; Zhang, H.; Podczerwinski, E.W. A reduction in triclosan and triclocarban in water resource recovery facilities’ influent, effluent, and biosolids following the US Food and Drug Administration’s 2013 proposed rulemaking on antibacterial products. Water Environ. Res. 2019, 91, 715–721.
- Food and Drug Administration. Safety and effectiveness of consumer antiseptics: Topical antimicrobial drug products for over-the-counter human use. Fed. Regist. 2016, 81, 61106–61130.
- Waidyanatha, S.; Black, S.R.; Patel, P.R.; Watson, S.L.; Snyder, R.W.; Sutherland, V.; Stanko, J.; Fennell, T.R. Disposition and metabolism of antibacterial agent, triclocarban, in rodents; a species and route comparison. Xenobiotica 2020, 50, 1469–1482.
- Juncker, J.-C. (Ed.) Commission Impementing Decision not Approving Triclosan as an Existing Active Substance for Use in Biocidal Products for Product-Type 1, 528/2012; Union European: Brussels, Belgium, 2016.
- Chen, J.; Meng, X.Z.; Bergman, A.; Halden, R.U. Nationwide reconnaissance of five parabens, triclosan, triclocarban and its transformation products in sewage sludge from China. J. Hazard. Mater. 2019, 365, 502–510.
- Costa, N.O.; Forcato, S.; Cavichioli, A.M.; Pereira, M.R.F.; Gerardin, D.C.C. In utero and lactational exposure to triclocarban: Age-associated changes in reproductive parameters of male rat offspring. Toxicol. Appl. Pharmacol. 2020, 115077.
- Ye, X.; Zhou, X.; Furr, J.; Ahn, K.C.; Hammock, B.D.; Gray, E.L.; Calafat, A.M. Biomarkers of exposure to triclocarban in urine and serum. Toxicology 2011, 286, 69–74.
- Schebb, N.H.; Inceoglu, B.; Ahn, K.C.; Morisseau, C.; Gee, S.J.; Hammock, B.D. Investigation of human exposure to triclocarban after showering and preliminary evaluation of its biological effects. Environ. Sci. Technol. 2011, 45, 3109–3115.
- Gao, C.-J.; Kannan, K. Phthalates, bisphenols, parabens, and triclocarban in feminine hygiene products from the United States and their implications for human exposure. Environ. Int. 2020, 136, 105465.
- De Bellis, M.; De Luca, A.; Rana, F.; Cavalluzzi, M.M.; Catalano, A.; Lentini, G.; Franchini, C.; Tortorella, V.; Conte Camerino, D. Evaluation of the pharmacological activity of the major mexiletine metabolites on skeletal muscle sodium currents. Br. J. Pharmacol. 2006, 149, 300–310.
- Schebb, N.H.; Franze, B.; Maul, R.; Ranganathan, A.; Hammock, B.D. In vitro glucuronidation of the antibacterial triclocarban and its oxidative metabolites. Drug Metab. Disposit. 2012, 40, 25–31.
- Armstrong, D.L.; Lozano, N.; Rice, C.P.; Ramirez, M.; Torrents, A. Degradation of triclosan and triclocarban and formation of transformation products in activated sludge using benchtop bioreactors. Environ. Res. 2018, 161, 17–25.
- Miller, T.R.; Colquhoun, D.R.; Halden, R.U. Identification of wastewater bacteria involved in the degradation of triclocarban and its non-chlorinated congener. J. Hazard. Mater. 2010, 183, 766–772.
- Pycke, B.F.G.; Roll, I.B.; Brownawell, B.J.; Kinney, C.A.; Furlong, E.T.; Kolpin, D.W.; Halden, R.U. Transformation products and human metabolites of triclocarban and triclosan in sewage sludge across the United States. Environ. Sci. Technol. 2014, 48, 7881–7890.
- Birch, C.G.; Hiles, R.A.; Eichhold, T.H.; Jeffcoat, A.R.; Handy, R.W.; Hill, J.M.; Willis, S.L.; Hess, T.R.; Wall, M.E. Biotransformation products of 3,4,4′-trichlorocarbanilide in rat, monkey, and man. Drug Metab. Dispos. 1978, 6, 169–176.
- Schebb, N.H.; Flores, I.; Kurobe, T.; Franze, B.; Ranganathan, A.; Hammock, B.D.; Teh, S. Bioconcentration, metabolism and excretion of triclocarban in larval Quart Medaka (Oryzias latipes). Aquat. Toxicol. 2011, 105, 448–454.
- Zhang, H.; Lu, Y.; Liang, Y.; Jiang, L.; Cai, Z. Triclocarban-induced responses of endogenous and xenobiotic metabolism in human hepatic cells: Toxicity assessment based on nontargeted metabolomics approach. J. Hazard. Mater. 2020, 392, 122475.
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014.
- O’Neill, J. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. Rev. Antimicrob Resist. 2014, 20, 1–16.
- Kim, S.A.; Rhee, M.S. Microbicidal effects of plain soap vs. triclocarban-based antibacterial soap. J. Hosp. Infect. 2016, 94, 276–280.
- US Food and Drug Administration. Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Overthe-Counter Human Use; Proposed Amendment of the Tentative Final Monograph; Reopening of Administrative Record; US Food and Drug Administration: Silver Spring, MD, USA, 2013. Available online: https://www.federalregister.gov/articles/2013/12/17/2013-29814/safetyand-effectiveness-of-consumer-antiseptics-topical-antimicrobialdrug-products-for (accessed on 15 April 2021).
- Pozzi, C.; Ferrari, S.; Cortesi, D.; Luciani, R.; Stroud, R.M.; Catalano, A.; Costi, M.P.; Mangani, S. The structure of Enterococcus faecalis thymidylate synthase provides clues about folate bacterial metabolism. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 1232–1241.
- Dahl, A.; Iversen, K.; Tonder, N.; Hoest, N.; Arpi, M.; Dalsgaard, M.; Chehri, M.; Soerensen, L.L.; Fanoe, S.; Junge, S. Prevalence of infective endocarditis in Enterococcus faecalis bacteremia. J. Am. Coll. Cardiol. 2019, 74, 193–201.
- Yu, J.J.; Manus, M.B.; Mueller, O.; Windsor, S.C.; Horvath, J.E.; Nunn, C.L. Antibacterial soap use impacts skin microbial communities in rural Madagascar. PLoS ONE 2018, 13, e0199899.
- Pujol, E.; Blanco-Cabra, N.; Julián, E.; Leiva, R.; Torrents, E.; Vázquez, S. Pentafluorosulfanyl-containing triclocarban analogs with potent antimicrobial activity. Molecules 2018, 23, 2853.
- Catalano, A.; Iacopetta, D.; Rosato, A.; Salvagno, L.; Ceramella, J.; Longo, F.; Sinicropi, M.S.; Franchini, C. Searching for small molecules as antibacterials: Non-cytotoxic diarylureas analogues of triclocarban. Antibiotics 2021, 10, 204.
- Chen, J.; Ki, C.A.; Gee, N.A.; Ahmed, M.I.; Duleba, A.J.; Zhao, L.; Gee, S.J.; Hammock, B.D.; Lasley, B.L. Triclocarban enhances testosterone action: A new type of endocrine disruptor? Endocrinology 2008, 149, 1173–1179.
- Duleba, A.J.; Ahmed, M.I.; Sun, M.; Gao, A.C.; Villanueva, J.; Conley, A.J.; Turgeon, J.L.; Benirschke, K.; Gee, N.A.; Chen, J.; et al. Effects of triclocarban on intact immature male rat: Augmentation of androgen action. Reprod. Sci. 2011, 18, 119–127.
- Cao, L.Y.; Xu, Y.H.; He, S.; Ren, X.M.; Yang, Y.; Luo, S.; Xie, X.D.; Luo, L. Antimicrobial triclocarban exhibits higher agonistic activity on estrogen-related receptor γ than triclosan at human exposure levels: A novel estrogenic disruption mechanism. Environ. Sci. Technol. Lett. 2020, 7, 434–439.
- Gálvez-Ontiveros, Y.; Páez, S.; Monteagudo-Sanchez, C.; Rivas, A. Endocrine disruptors in food: Impact on gut microbiota and metabolic diseases: A systematic review. Nutrients 2020, 12, 1158.
- Rochester, J.R.; Bolden, A.L.; Pelch, K.E.; Kwiatkowski, C.F. Potential developmental and reproductive impacts of triclocarban: A scoping review. J. Toxicol. 2017, 2017, 9679738.
- Aker, A.M.; Ferguson, K.K.; Rosario, Z.Y.; Mukherjee, B.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. The associations between prenatal exposure to triclocarban, phenols and parabens with gestational age and birth weight in northern Puerto Rico. Environ. Res. 2019, 169, 41–51.
- Watkins, D.J.; Ferguson, K.K.; Anzalota Del Toro, L.V.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int. J. Hyg. Environ. Health 2015, 218, 212–219.
- Wei, J.; Zhou, T.; Hu, Z.; Li, Y.; Yuan, H.; Zhao, K.; Zhang, H.; Liu, C. Effects of triclocarban on oxidative stress and innate immune response in zebrafish embryos. Chemosphere 2018, 210, 93–101.
- Ding, Z.M.; Ahmad, M.J.; Meng, F.; Chen, F.; Wang, Y.S.; Zhao, X.Z.; Zhang, S.X.; Miao, Y.L.; Xiong, J.J.; Huo, L.J. Triclocarban exposure affects mouse oocyte in vitro maturation through inducing mitochondrial dysfunction and oxidative stress. Environ. Pollut. 2020, 262, 114271.
- Xie, M.; Zhang, H.; Wang, W.; Sherman, H.L.; Minter, L.M.; Cai, Z.; Zhang, G. Triclocarban Exposure Exaggerates Spontaneous Colonic Inflammation in Il-10−/− Mice. Toxicol. Sci. 2020, 174, 92–99.
- Dong, M.; Yuan, P.; Song, Y.; Lei, H.; Chen, G.; Zhu, X.; Wu, F.; Chen, C.; Liu, C.; Shi, Z.; et al. In vitro effects of triclocarban on adipogenesis in murine preadipocyte and human hepatocyte. J. Hazard. Mater. 2020, 122829.
- Li, H.; Zhao, Y.; Chen, L.; Su, Y.; Li, X.; Jin, L.; Ge, R.S. Triclocarban and triclosan inhibit human aromatase via different mechanisms. BioMed Res. Int. 2017, 2017, 8284097.
- Taweetanawanit, P.; Ratpukdi, T.; Siripattanakul-Ratpukdi, S. Performance and kinetics of triclocarban removal by entrapped Pseudomonas fluorescens strain MC46. Bioresour. Technol. 2019, 274, 113–119.
- Kennedy, R.C.; Fling, R.R.; Robeson, M.S.; Saxton, A.M.; Donnell, R.L.; Darcy, J.L.; Bemis, D.A.; Liu, J.; Zhao, L.; Chen, J. Temporal development of gut microbiota in triclocarban exposed pregnant and neonatal rats. Sci. Rep. 2016, 6, 33430.
- Ribado, J.V.; Ley, C.; Haggerty, T.D.; Tkachenko, E.; Bhatt, A.S.; Parsonnet, J. Household triclosan and triclocarban exposure impacts the adult intestinal microbiome but not the infant intestinal microbiome. BioRxiv 2017, 126334.
- Pflughoeft, K.J.; Versalovic, J. Human microbiome in health and disease. Annu. Rev. Pathol. 2012, 7, 99–122.
