Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Barbara Elisabeth Teixeira-Costa and Version 2 by Conner Chen.
Chitosan is a versatile biomolecule with a broad range of applications in food and pharmaceutical products. It can be obtained by the alkaline deacetylation of chitin. This biomolecule can be extracted using conventional or green methods from seafood industry residues, e.g., shrimp shells. Chitin has limited applications because of its low solubility in organic solvents. Chitosan is soluble in acidified solutions allowing its application in the food industry. Furthermore, biological properties, such as antioxidant, antimicrobial, as well as its biodegradability, biocompatibility and nontoxicity have contributed to its increasing application as active food packaging.
- chitosan
- seafood industry residues
- crustacean shells
- green solvents
- plasticizer
- biodegradable food packaging
- coatings
- polysaccharides
- Chitin
1. Food Packaging
Packaging protects foods from the environment. Over the years, packages have changed to follow the different demands of consumers and the evolution of the food industry. Packaging is responsible for maintaining food quality, improving safety and shelf life, as well as providing label information of ingredients, and promoting the worldwide distribution of food products to reach final consumers [1][2]. From all of these, the maintenance of food quality across the whole supply chain is the most critical factor when choosing a packaging material [3][4]. To fulfill these functions, the materials for the packaging design must have good thermo-mechanical properties, to be properly processed by different means, resist the surrounding aspects through distribution, exhibit an adequate shelf life for product storage and prevent cross contamination of unwanted substances to their content [5]. Moreover, packaging technology is dynamic and has evolved since ancient times until nowadays changing humans’ lifestyle. Through this timeline, different materials have been used for food packaging, such as glass, metal, paper, and plastic, though these last two are more often utilized for this purpose [1][6][7].
Conventional plastic packaging made from synthetic polymers are items of one-time use, generally discarded in the environment after using. Around 40% of petrochemical-based plastics are used for packaging purposes and close to 60% of plastic packaging are used to pack food and beverages [8]. About 95% of plastic packaging are wasted after a single-use cycle and end up in the environment, where it can be broken down into micro or nanoplastics [9][10]. These microplastics are carried out by ocean currents and can be ingested by the sea-wild life and birds, in which they accumulate in their digestive system, modify their health, and end up in the food chain [11].
Another issue that negatively affects plastic packaging production is concerns about human occupational health, since different hazardous chemicals added intentionally and unintentionally are used during processing [8]. Moreover, during the manufacturing of synthetic petroleum-based plastics, greenhouse gases and particulates are released in the environment, also contributing to world pollution [5]. In addition to all of this, packaging manufacturers face a new market challenge due to consumer demand for biobased resources for food packaging [5][12]. Thus, all these circumstances have been stimulating the search for new biobased materials and the development of biodegradable packaging from renewable sources as strategies to mitigate pollution and sustain the planet. In response to these challenges, there is a great opportunity for the development of novel food packaging. In this context, biobased, biodegradable, compostable, edible, active and intelligent packaging are innovative trends in the field.
2. Chitosan-Based Films to Food Packaging
Chitosan is among the most studied polysaccharides for the development of film/coating packaging. This is justified by its versatility, good physical-chemical and biological properties. Mechanical properties are affected by the chitosan DA, solvent, pH, concentration, viscosity, and molecular weight [1]. Moreover, chitosan is generally less expensive and commercially available when compared to other biopolymers [13]. The first reports of chitosan films are from the mid-1930s, when George W. Rigby patented it [14]. Since then, the employment of chitosan as films, coatings, or composites, to different products has been extensive. Recent works on the sustainable application of chitosan-based films for food packaging were reviewed by Haghighi et al. [3] and Mujtaba et al. [1].
As with any other packaging, edible films and coatings must protect the food integrity, maintaining quality, controlling mass and volatile losses, extending its shelf life, preventing surface contamination, offering mechanical protection, and improving its sensory properties (Figure 1) [3][15][16][17]. Although, it is not expected that edible films can completely replace all conventional packaging, they can be used to significantly reduce the use of petroleum-based plastics, decrease food losses, and reduce the environment pollution in the long-term. Edible films and coatings may have in their composition two main based materials, a biomacromolecule-based matrix, and additives, such as plasticizers, cross-linkers, other reinforcements substances and functional ingredients [17]. These materials can be used alone or blended. As edible packaging is composed of natural-based materials, it can be classified as a subgroup of biobased and biodegradable packaging [16]. Generally, polysaccharides, proteins, lipids, and their mixtures are used for the development of edible films and coatings [17].
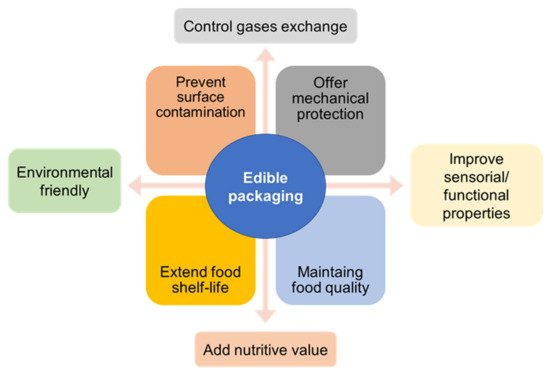
Figure 1. Main purposes of an edible food packaging.
Films based on chitosan are environmental-friendly materials and can be degraded by microorganisms [18]. Leceta et al. [19] have studied the environmental assessment between two different food packaging systems, polypropylene, and chitosan films, during different life cycle stages. For them, from about 1 kg of chitosan-based film waste, 20% can be considered as compost and the remaining 80% degraded as organic matter by microorganisms. Furthermore, these authors found that the highest positive impact of chitosan-based films was related to the end-of-life scenarios, especially associated with composting and carcinogens, when compared to polypropylene films, which can provide a reduction of environmental pollution generated by the food packaging industry.
In another work, the biodegradability of chitosan-based films on three different soils, industrial compost, commercial garden soil, and soil from a vineyard, was evaluated. The authors found that the properties of the chitosan-based film deteriorated in less than 3 days and its biodegradation occurred in all tested soils after 14 days [20]. Moreover, when these films were incorporated with Quercus polyphenol extract its active properties in compost and garden soil occurred in 6 days, whereas the total biodegradation process was not completed in the vineyard soil during the 14 days. These authors also found that adding water to the soil decreased the rate of biodegradation from the chitosan film on the terrestrial environment. These findings are relevant to confirm the biodegradability of chitosan films when using it as a sustainable alternative food packaging.
Pure chitosan films can only be prepared via solvent casting. In this process, chitosan is dissolved using enough solvent, mainly, acidified water, placed on a flat surface and let to dry until constant weight. Generally, these pure films are reported to exhibit a smooth, continuous, and compact surface, but are brittle and fragile possessing low mechanical properties [21][22][23]. To overcome the low mechanical performance and high sensitivity to water, the addition of plasticizers and other different approaches have been indicated. Some of these use crosslinking, complexation, graft copolymerization, surface coating, filler incorporation and others [3]. Blending chitosan with other polymers by solution or extrusion blending to form composite films could also be a strategy. Binary or ternary blends, e.g., CS-gelatin, CS-alginate, CS-gelatin-caprolactone, have been developed for food and biomedical applications [22]. The formation of a cohesive film-forming material resistant to rupture between CS and cellulose in the paper industry have also been reported, as the wet strength of paper can be improved by CS addition [22]. In another study, CS was used as a surface coating agent on printing paper, improving its mechanical properties, such as its strength, and inhibited the growth of bacteria, such as E. coli [24].
Regarding the mechanical properties, tensile strength (TS) and elongation at break (EB) are two of the most important characteristics for film applications [13]. TS is related to the maximum tensile stress that a film can hold up [25]. Moreover, the TS of chitosan films with high and low molecular weight was described as dependent on storage time due to the conformational changes of chitosan molecules by the free volume reduction [26]. The type of solvent acid used to prepare chitosan-starch composite films also influenced TS according to the following order: lactic acid < malic < acetic acid [27]. In another work, chitosan film displayed a significantly lower TS, 18.252 MPa, but a higher EB, ~40%, than gelatin and composite films [28]. In the development of chitosan-zein composite films, Sun et al. [25] found that TS was influenced by different plasticizers, sorbitol < glycerol and PEG-400; this result was explained by the number of hydroxyl groups in the plasticizer. Sorbitol has the capacity of binding a higher number of water molecules. The TS values may also decrease with the increase in pH because of a lower chitosan dissociation [6]. Furthermore, in chitosan composite films with quinoa protein, it was observed that TS and EB were influenced by the presence of protein in the films [29]. Some application examples of chitosan-based films are listed in Table 1.
Table 1. Broad application of chitosan-based films and their main results.
| Films Matrix | Composite Ingredients | Applications | Main Results | References | ||||
|---|---|---|---|---|---|---|---|---|
| Chitosan/Zein | Glycerol, PEG-400, and sorbitol | Potential food application | Permeability increased with higher plasticizer concentration. PEG-400 promoted a better barrier property. |
[25] | ||||
| Chitosan | Aloe vera gel and silver nanoparticles (SNPs) | Potential biomedical applications | SNPs decrease the crystallinity of the films. SNPs affected the structure and viscoelastic behavior of chitosan films. |
[30] | ||||
| Chitosan | Glycerol | Strawberries | Protection of the fruit against fungi. Maintenance of flavor, appearance, texture, and aroma. |
[31] | ||||
| Chitosan/purple yam starch | Glycerol | Coating of apples | The films preserved the quality of apples for four weeks of storage. Weight loss from the coated apples was less significant than the uncoated. |
[32] | ||||
| Chitosan | Apple peel polyphenols (APP) | Potential bio-composite food packaging material | APP significantly increased thickness, density, solubility, opacity, and swelling ratio of films. APP decreased the moisture content, water vapor permeability, tensile strength and elongation at break of the films. |
[33] | ||||
| Chitosan/organoclay nanocomposites (OrgMMT) | Olive oil and corn oil as plasticizers | Potential food packaging applications | OrgMMT significantly reduced the elongation at break of all oil containing samples, acting as stress concentrator upon deformation. Corn oil was a less effective as plasticizer than olive oil. |
[34] | ||||
| Chitosan/gelatin | Red grape seed extract and | Ziziphora clinopodioides | essential oil | Potential food packaging applications | The addition of red grape seed extract and | Z. clinopodioides | essential oil improved total phenolic content, antibacterial and antioxidant activities, thickness, and water vapor barrier property. | [35] |
| Chitosan/corn starch | Glycerol as plasticizer | Potential food/pharmaceutical applications | The water vapor permeability and moisture content of films increased with an increase in chitosan concentration. | [36] | ||||
| Chitosan hydrochloride (CHC) | Glycerol as plasticizer and epigallocatechin gallate (EGCG) nanocapsules (NCs) | Potential food packaging applications | The incorporation of nanocapsules into the CHC films increased their tensile strength and percentage of elongation at break. The produced films could prevent lipids oxidation of fatty food products |
[18] | ||||
| Chitosan | Propolis extract | Potential food packaging applications | All chitosan/Propolis films inhibited bacteria on contact surface underneath the film. Mechanical properties, oxygen and moisture barrier, antioxidant and antimicrobial properties were improved by the addition of Propolis extract into the films. |
[37] | ||||
| Chitosan | Clove oil ( | Syzygium aromaticum | ) | Cooked pork sausages | The shelf life of cooked pork sausages increased from 14 to 20 days with the chitosan/clove oil films. | [38] | ||
| Chitosan/gelatin | Glycerol as plasticizer | Beef steaks | Myoglobin oxidation during retail display was reduced and the percentage of deoxymyoglobin increased with gelatin content in films. | [39] | ||||
| Chitosan | Glycerine, chokeberry pomace extract | Potential food packaging applications | Enhanced water vapor permeability and reduced oxygen permeability by addition of chokeberry extracts. The films showed significant antioxidant properties. | [40] | ||||
| Chitosan | Urtica dioica | leaf extract derived copper oxide (CuO) and zinc oxide (ZnO) nanoparticles | Food film packaging and shelf life of guava fruit | Low antioxidant activity and greater antimicrobial properties. Decreased moisture content, water holding capacity, and solubility. The nanocomposite chitosan films improved the quality and shelf-life attributes of guava by one week when compared to unpackaged fruits. |
[41] | |||
| Multilayer chitosan composites films | Sodium sulfoethyl cellulose (SEC), sodium alginate (ALG), and sodium hyaluronate (HA) | Potential biomedical and food applications | Effect of the polyelectrolyte complex layer on the properties of the poly-layer composites decreases in the order CS/SEC > CS/HA > CS/ALG. | [42] |
Water vapor and the oxygen barrier are relevant factors to consider in the development of food packaging since both can influence the food quality and shelf life. The evaluation of water vapor permeability (WVP) of polysaccharide-based films provides information of diffusion and solubility of water molecules through the polymeric matrix and will be influenced by its composition. In this context, the WVP of high and low molecular weight chitosan films was investigated by Kerch and Korkhov [26]. They found that the high molecular weight chitosan films exhibited higher permeability. The addition of a natural phenolic antioxidant, protocatechuic acid, significantly decreased the WVP of chitosan composite films [43]. Furthermore, the addition of vegetable olive and corn oils displayed a significant effect decreasing the WVP by reducing the hydrophilic content of the film [34]. Different mechanical and barrier properties of chitosan films have been reported. These properties are affected by chitosan molecular weight, solvent, plasticizer content, film composition, and pH, which makes the comparison between them more challenging.
3. Plasticizing Biodegradable Films with Green Solvents
In the development of biobased films, the hydrophilic character of their natural macromolecules matrix should be considered since water molecules may be a problem for the shelf life of the product as well as the physical stability of the packaging. To overcome this issue, hydrophobic or less hydrophilic substances can be incorporated to the films modifying the physical properties. These substances are denominated plasticizers and are generally of low molecular weight. Plasticizers are responsible for increasing the matrix free volume and molecular mobility to amorphous biopolymers, competing chain-to-chain H-bonding along the polymer chains [44][45]. They are often used to reduce the brittleness by reducing the polymer interchain interactions by putting themselves between polymer molecules, although they do not chemically bind to it [17].
The most commonly used plasticizers are glycerol, sorbitol, polyethylene glycols (PEG), lipids and their derivatives, such as fatty acids, phospholipids, lecithin, oils, and waxes, low molecular weight sugars, e.g., fructose-glucose syrups and honey, and others [44][17][23]. Crosslinkers and micro/nano-reinforcements can be incorporated into film packaging aiming superior tensile, gas and water barrier properties. The improvement of coatings cohesive strength can be achieved by application of other physical treatments, i.e., irradiation, by crosslinking formation [44]. More recently, films’ plasticization can be performed using deep eutectic solvents (DES), as sustainable solvent systems [46][47][48].
DESs are obtained by the complexation of a hydrogen bond donor (HBD) with a quaternary ammonium salt (hydrogen bond acceptor—HBA), producing liquids with physical and solvent properties analogue to ionic liquids (ILs) [49][50]. Ionic liquids are liquid solvents with a melting point below 100 °C and are formed from systems composed primarily of one type of discrete anion and cation. Initially, DESs were extensively used to decrease the temperature of molten salt. Nowadays, they have been used for a wide range of applications, from lubrication of steel to synthesizing drugs [50][51].
DESs contain large asymmetric ions of the HBA that have low lattice energy and so, low melting points, caused by hydrogen bonding formation. A wide range of mixtures of substances can form DES, which was critically reviewed by Smith et al. [50]. For plasticizing film packaging the most common DES used are the mixture of choline chloride-citric acid (ChCl-CA), choline chloride-urea (ChCl-Urea) and choline chloride-glycerol (ChCl-Gly). The latter has been used mainly for chitosan-based coatings [46][47][52]. Particularly, the eutectic mixtures of ChCl:Gly at a molar ratio of 1:2 were found to efficiently plasticize starch and starch/zein blends, and chitosan and chitosan/micro-crystalline cellulose/curcumin composites [47][48].
Many advantages can be pointed out from using DES as they are much cheaper, safer and easier to manufacture than ionic liquids. Moreover, DES are chemically and thermally stable, non-flammable, possessing high dissolution ability, lack of flammability, low volatility, low melting point and tailorability, as well as being water neutral, and low- or non-toxic. The DES toxicity, including cytotoxicity and phytotoxicity, is highly dependent on the testing species used on the mixture and their responses, as well as their physicochemical properties, concentration, and salts counteranion species [52]. In the work of Mbous et al. [53], the toxicity of some DES was tested, and they found that pure ChCl exhibited lower cytotoxic values than its aqueous solutions (EC50 30 ≥ ChClaq ≤ 40 mM), suggesting that ChCl did not dissociate after crossing the cellular membrane, implying a nontoxic profile of ChCl-based DES, although more in vivo or in vitro studies could be further investigated [53]. As they are often biodegradable, they can be considered green solvents. DESs are appropriate for biobased packaging processing and application. For the preparation of polysaccharide films, the DES mixture should be separately prepared and incorporated to the matrix materials or can be formed in situ after heat homogenization with other components [52].
References
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904.
- Ribeiro-Santos, R.; Andrade, M.; Melo, N.R.; de Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140.
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551–100567.
- Sohail, M.; Sun, D.-W.; Zhu, Z. Recent developments in intelligent packaging for enhancing food quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 2650–2662.
- Kamdem, D.P.; Shen, Z.; Nabinejad, O. Development of biodegradable composite chitosan-based films incorporated with xylan and carvacrol for food packaging application. Food Packag. Shelf Life 2019, 21, 100344–100366.
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as a preservative for fruits and vegetables: A review on chemistry and antimicrobial properties. J. Bioresour. Bioprod. 2019, 4, 11–21.
- Carina, D.; Sharma, S.; Jaiswal, A.K.; Jaiswal, S. Seaweeds polysaccharides in active food packaging: A review of recent progress. Trends Food Sci. Technol. 2021, 110, 559–572.
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019, 651, 3253–3268.
- Kleine Jäger, J.; Piscicelli, L. Collaborations for circular food packaging: The set-up and partner selection process. Sustain. Prod. Consum. 2021, 26, 733–740.
- Soares, J.; Miguel, I.; Venâncio, C.; Lopes, I.; Oliveira, M. Public views on plastic pollution: Knowledge, perceived impacts, and pro-environmental behaviours. J. Hazard. Mater. 2021, 412, 125227–125235.
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739–124751.
- Flury, M.; Narayan, R. Biodegradable plastic as an integral part of the solution to plastic waste pollution of the environment. Curr. Opin. Green Sustain. Chem. 2021, 30, 100490.
- Van den Broek, L.A.M.; Knoop, R.J.I.; Kappen, F.H.J.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242.
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643.
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables-A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169.
- Petkoska, A.T.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Res. Int. 2021, 140, 109981.
- Mkandawire, M.; Aryee, A.N. Resurfacing and modernization of edible packaging material technology. Curr. Opin. Food Sci. 2018, 19, 104–112.
- Liang, J.; Yan, H.; Zhang, J.; Dai, W.; Gao, X.; Zhou, Y.; Wan, X.; Puligundla, P. Preparation and characterization of antioxidant edible chitosan films incorporated with epigallocatechin gallate nanocapsules. Carbohydr. Polym. 2017, 171, 300–306.
- Leceta, I.; Guerrero, P.; Cabezudo, S.; de la Caba, K. Environmental assessment of chitosan-based films. J. Clean. Prod. 2013, 41, 312–318.
- Oberlintner, A.; Bajić, M.; Kalčíková, G.; Likozar, B.; Novak, U. Biodegradability study of active chitosan biopolymer films enriched with Quercus polyphenol extract in different soil types. Environ. Technol. Innov. 2021, 21, 101318.
- Hoell, I.A.; Vaaje-Kolstad, G.; Eijsink, V.G.H. Structure and function of enzymes acting on chitin and chitosan. Biotechnol. Genet. Eng. Rev. 2010, 27, 331–366.
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368.
- Liu, M.; Zhou, Y.; Zhang, Y.; Yu, C.; Cao, S. Preparation and structural analysis of chitosan films with and without sorbitol. Food Hydrocoll. 2013, 33, 186–191.
- Zakaria, S.; Chia, C.H.; Wan Ahmad, W.H.; Kaco, H.; Chook, S.W.; Chan, C.H. Mechanical and Antibacterial Properties of Paper Coated with Chitosan. Sains Malays. 2015, 44, 905–911.
- Sun, Y.; Liu, Z.; Zhang, L.; Wang, X.; Li, L. Effects of plasticizer type and concentration on rheological, physico-mechanical and structural properties of chitosan/zein film. Int. J. Biol. Macromol. 2020, 143, 334–340.
- Kerch, G.; Korkhov, V. Effect of storage time and temperature on structure, mechanical and barrier properties of chitosan-based films. Eur. Food Res. Technol. 2011, 232, 17–22.
- Zhong, Y.; Li, Y. Effects of storage conditions and acid solvent types on structural, mechanical and physical properties of kudzu starch (Pueraria lobata)-chitosan composite films. Starch Stärke 2011, 63, 579–586.
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible films from chitosan-gelatin: Physical properties and food packaging application. Food Biosci. 2021, 40, 100871.
- Abugoch, L.E.; Tapia, C.; Villamán, M.C.; Yazdani-Pedram, M.; Díaz-Dosque, M. Characterization of quinoa protein-chitosan blend edible films. Food Hydrocoll. 2011, 25, 879–886.
- Madian, N.G.; Mohamed, N. Enhancement of the dynamic mechanical properties of chitosan thin films by crosslinking with greenly synthesized silver nanoparticles. J. Mater. Res. Technol. 2020, 9, 12970–12975.
- Pavinatto, A.; de Almeida Mattos, A.V.; Malpass, A.C.G.; Okura, M.H.; Balogh, D.T.; Sanfelice, R.C. Coating with chitosan-based edible films for mechanical/biological protection of strawberries. Int. J. Biol. Macromol. 2020, 151, 1004–1011.
- Da Costa, J.C.M.; Miki, K.S.L.; da Ramos, A.S.; Teixeira-Costa, B.E. Development of biodegradable films based on purple yam starch/chitosan for food application. Heliyon 2020, 6, 1–10.
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555.
- Giannakas, A.; Patsaoura, A.; Barkoula, N.-M.; Ladavos, A. A novel solution blending method for using olive oil and corn oil as plasticizers in chitosan based organoclay nanocomposites. Carbohydr. Polym. 2017, 157, 550–557.
- Shahbazi, Y. The properties of chitosan and gelatin films incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil as biodegradable materials for active food packaging. Int. J. Biol. Macromol. 2017, 99, 746–753.
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643.
- Siripatrawan, U.; Vitchayakitti, W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016, 61, 695–702.
- Lekjing, S. A chitosan-based coating with or without clove oil extends the shelf life of cooked pork sausages in refrigerated storage. Meat Sci. 2016, 111, 192–197.
- Cardoso, G.P.; Dutra, M.P.; Fontes, P.R.; Ramos, A.D.L.S.; Gomide, L.A.D.M.; Ramos, E.M. Selection of a chitosan gelatin-based edible coating for color preservation of beef in retail display. Meat Sci. 2016, 114, 85–94.
- Sady, S.; Błaszczyk, A.; Kozak, W.; Boryło, P.; Szindler, M. Quality assessment of innovative chitosan-based biopolymers for edible food packaging applications. Food Packag. Shelf Life 2021, 30, 100756.
- Kalia, A.; Kaur, M.; Shami, A.; Jawandha, S.K.; Alghuthaymi, M.A.; Thakur, A.; Abd-Elsalam, K.A. Nettle-Leaf Extract Derived ZnO/CuO Nanoparticle-Biopolymer-Based Antioxidant and Antimicrobial Nanocomposite Packaging Films and Their Impact on Extending the Post-Harvest Shelf Life of Guava Fruit. Biomolecules 2021, 11, 224.
- Gubanova, G.N.; Petrova, V.A.; Kononova, S.V.; Popova, E.N.; Smirnova, V.E.; Bugrov, A.N.; Klechkovskaya, V.V.; Skorik, Y.A. Thermal properties and structural features of multilayer films based on chitosan and anionic polysaccharides. Biomolecules 2021, 11, 762.
- Liu, J.; Liu, S.; Wu, Q.; Gu, Y.; Kan, J.; Jin, C. Effect of protocatechuic acid incorporation on the physical, mechanical, structural and antioxidant properties of chitosan film. Food Hydrocoll. 2017, 73, 90–100.
- Quirós-Sauceda, A.E.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Edible coatings as encapsulating matrices for bioactive compounds: A review. J. Food Sci. Technol. 2014, 51, 1674–1685.
- Rivero, S.; Damonte, L.; Garcia, M.A.; Pinotti, A. An Insight into the Role of Glycerol in Chitosan Films. Food Biophys. 2016, 11, 117–127.
- Almeida, C.M.R.; Magalhães, J.M.C.S.; Souza, H.K.S.; Gonçalves, M.P. The role of choline chloride-based deep eutectic solvent and curcumin on chitosan films properties. Food Hydrocoll. 2018, 81, 456–466.
- Pereira, P.F.; Andrade, C.T. Optimized pH-responsive film based on a eutectic mixture-plasticized chitosan. Carbohydr. Polym. 2017, 165, 238–246.
- Sousa, A.M.M.; Souza, H.K.S.; Latona, N.; Liu, C.-K.; Gonçalves, M.P.; Liu, L. Choline chloride based ionic liquid analogues as tool for the fabrication of agar films with improved mechanical properties. Carbohydr. Polym. 2014, 111, 206–214.
- Abbott, A.P.; Harris, R.C.; Ryder, K.S.; D’Agostino, C.; Gladden, L.F.; Mantle, M.D. Glycerol eutectics as sustainable solvent systems. Green Chem. 2011, 13, 82–90.
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082.
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R. Deep Eutectic Solvents Formed Between Choline Chloride and Carboxylic Acids. J. Am. Chem. Soc. 2004, 126, 9142.
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing. A review. Carbohydr. Polym. 2018, 200, 361–380.
- Mbous, Y.P.; Hayyan, M.; Wong, W.F.; Looi, C.Y.; Hashim, M.A. Unraveling the cytotoxicity and metabolic pathways of binary natural deep eutectic solvent systems. Sci. Rep. 2017, 7, 41257.
More
