The photooxidative degradation process of plastics caused by ultraviolet irradiation leads to bond breaking, crosslinking, the elimination of volatiles, formation of free radicals, and decreases in weight and molecular weight. Photodegradation deteriorates both the mechanical and physical properties of plastics and affects their predicted life use, in particular for applications in harsh environments. Plastics have many benefits, while on the other hand, they have numerous disadvantages, such as photodegradation and photooxidation in harsh environments and the release of toxic substances due to the leaching of some components, which have a negative effect on living organisms. Therefore, attention is paid to the design and use of safe, plastic, ultraviolet stabilizers that do not pose a danger to the environment if released. Plastic ultraviolet photostabilizers act as efficient light screeners (absorbers or pigments), excited-state deactivators (quenchers), hydroperoxide decomposers, and radical scavengers. Ultraviolet absorbers are cheap to produce, can be used in low concentrations, mix well with polymers to produce a homogenous matrix, and do not alter the color of polymers.
- plastics
- polyvinyl chloride
- photostabilizers
- plastic photodegradation and photooxidation
- recycling of plastics
- photoirradiation
1. Introduction
| Plastic (Repeating Unit) | Name | European Demand (%) | |
|---|---|---|---|
| C–C Backbone | 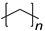 |
PE | 29.6 |
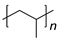 |
PP | 19.9 | |
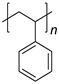 |
PS | 7.1 | |
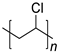 |
PVC | 10.4 | |
| Heteroatoms in backbone | 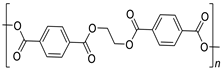 |
PET | 6.9 |
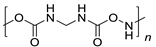 |
PU | 7.4 | |
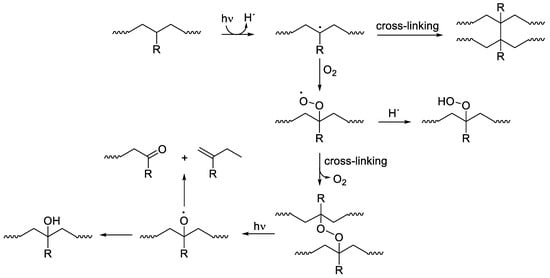
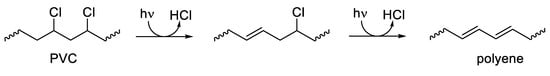
2. Photostabilization of Polymers Using UV Absorbers
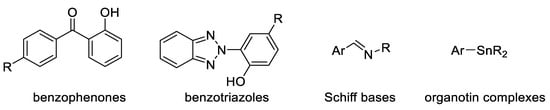
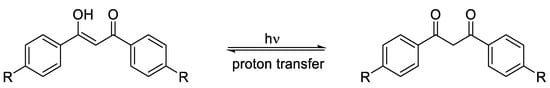
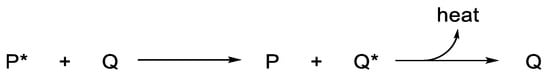
In conclusion, polymer stabilization is one of the most important processes that is used to elongate the lifetimes of plastic products. Plastics used in outdoor applications suffer in harsh environments and quickly lose their mechanical and physical properties. The proper solution for inhibiting the photooxidation of plastics due to the inevitable exposure to light and oxygen is through the addition of efficient ultraviolet absorbers that are capable of acting as efficient scavengers for light and blocking the formation of free radicals within the polymeric chains. The additives should absorb irradiation light directly and decompose peroxide species. In addition, they should be very compatible with the polymers, not alter the color, be used at a very low concentration, and be safe for the environment if released. Progress was made with the design and use of safe additives to enhance plastic stability and, in particular, polystyrene and polyvinyl chloride. Polyphosphates, Schiff bases, and organometallic complexes containing aromatic moieties showed the potential to be used as ultraviolet absorbers for plastics. The damage on the surface of irradiated plastics in the presence of ultraviolet absorbers is low compared with the blank films.
Since the additives are not linked to plastic through covalent bonds, they can be leached to the surrounding environments. The leakage of these chemicals followed by their degradation poses a danger to both animals and humans. Therefore, future research should be attention to the design, synthesis, and use of safe, non-toxic, and highly stable polymeric additives to suppress the degradation of plastic. Some progress was made, but there is still room for further improvements and modifications.- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus 2013, 2, 398, doi:10.1186/2193-1801-2-398.
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511, doi:10.1021/acssuschemeng.9b06635.
- Yaqoob, A.A.; Noor, N.H.M.; Serrà, A.; Mohamad Ibrahim, M.N. Advances and challenges in developing efficient graphene oxide-based ZnO photocatalysts for dye photo-oxidation. Nanomaterials 2020, 10, 932, doi:10.3390/nano10050932.
- The State of Plastics: World Environment Day Outlook 2018. Available online: https://www.unep.org/resources/report/state-plastics-world-environment-day-outlook-2018 (accessed on 5 November 2021).
- Plastics—The Facts 2020. An Analysis of European Plastics Production, Demand and Waste Data. Available online: https://issuu.com/plasticseuropeebook/docs/plastics_the_facts-web-dec2020 (accessed on 2 November 2021).
- Wheeler, R.N., Jr. Poly (vinyl chloride) processes and products. Health Perspect. 1981, 41, 123–128, doi:10.1289/ehp.8141123.
- Schyns, Z.O.G.; Shaver, M.M. Mechanical recycling of packaging plastics: A review. Rapid Commun. 2021, 42, 2000415, doi:10.1002/marc.202000415.
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of plastic solid waste: A state of art review and future applications. B. Eng. 2017, 115, 409–422, doi:10.1016/j.compositesb.2016.09.013.
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Sci. Process. Impacts 2015, 17, 1513–152, doi:10.1039/C5EM00207A.
- Matthews, C.; Moran, F.; Jaiswal, A.K. A review on European Union’s strategy for plastics in a circular economy and its impact on food safety. Clean. Prod. 2021, 283, 125263, doi:10.1016/j.jclepro.2020.125263.
- Potaufeux, J.-E.; Odent, J.; Notta-Cuvier, D.; Lauro, F.; Raquez, J.-M. A comprehensive review of the structures and properties of ionic polymeric materials. Chem. 2020, 11, 5914–5936, doi:10.1039/D0PY00770F.
- Moretti, E.; Zinzi, M.; Belloni, E. Polycarbonate panels for buildings: Experimental investigation of thermal and optical performance. Energy Build. 2014, 70, 23–35, doi:10.1016/j.enbuild.2013.11.04.
- Turner, A.; Filella, M. Polyvinyl chloride in consumer and environmental plastics, with a particular focus on metal-based additives. Sci. Process. Impacts 2021, 23, 1376–1384, doi:10.1039/D1EM00213A.
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Adv. 2008, 26, 246–265, doi:10.1016/j.biotechadv.2007.12.005.
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. Hazard. Mater. 2018, 344, 179–199, doi:10.1016/j.jhazmat.2017.10.014.
- Umar, K.; Yaqoob, A.A.; Ibrahim, M.N.M.; Parveen, T.; Safian, M.T. Environmental applications of smart polymer composites. Smart Polym. Nanocompos. Biomed. Environ. Appl. 2020, 15, 295–320, doi:10.1016/B978-0-12-819961-9.00008-6.
- Albertsson, A.-C.; Karlsson, S. The three stages in degradation of polymers—Polyethylene as a model substance. Appl. Polym. Sci. 1988, 35, 1289–1302, doi:10.1002/app.1988.070350515.
- Chatge, S.; Yang, Y.; Ahn, J.-H.; Hur, H.-G. Biodegradation of polyethylene: A brief review. Biol. Chem. 2020, 63, 27, doi:10.1186/s13765-020-00511-3.
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Degrad. Stab. 2008, 93, 561–584, doi:10.1016/j.polymdegradstab.2007.11.008.
- Vohlídal, J. Polymer degradation: A short review. Teach. Int. 2020, 3, 213–220, doi:10.1515/cti-2020-0015.
- Gryn’ova, G.; Hodgson, J.L.; Coote, M.L. Revising the mechanism of polymer autooxidation. Biomol. Chem. 2011, 9, 480–490, doi:10.1039/C0OB00596G.
- Webb, H.K.; Arnott, J.; Crawford, R.J.; Ivanova, E.P. Plastic degradation and its environmental implications with special reference to poly (ethylene terephthalate). Polymers 2013, 5, 1–18, doi:10.3390/polym5010001.
- Raquez, J.M.; Bourgeois, A.; Jacobs, H.; Degée, P.; Alexandre, M.; Dubois, P. Oxidative degradations of oxodegradable LDPE enhanced with thermoplastic pea starch: Thermo mechanical properties, morphology, and UV-ageing studies. Appl. Polym. Sci. 2011, 122, 489–496, doi:10.1002/app.34190.
- Zheng, Y.; Yanful, E.K.; Bassi, A.S. A review of plastic waste biodegradation. Rev. Biotechnol. 2005, 25, 243–250, doi:10.1080/07388550500346359.
- Müller, R.-J.; Kleeberg, I.; Deckwer, W.-D. Biodegradation of polyesters containing aromatic constituents. Biotechnol. 2001, 86, 87–95, doi:10.1016/S0168-1656(00)00407-7.
- Andrady, A.L. Microplastics in the marine environment. Pollut. Bull. 2011, 62, 1596–1605, doi:10.1016/j.marpolbul.2011.05.030.
- Coyle, R.; Hardiman, G.; O’Driscoll, K. Microplastics in the marine environment: A review of their sources, distribution processes, uptake and exchange in ecosystems. Case Stud. Therm. Environ. Eng. 2020, 2, 100010, doi:10.1016/j.cscee.2020.100010.
- Paulsson, M.; Parkås, J. Review: Light-induced yellowing of lignocellulosic pulps–mechanism and penetrative methods. BioResources 2012, 7, 5995–6040, doi:10.15376/biores.7.4.5995-6040.
- Jin, C.; Christensen, P.A.; Egerton, T.A.; Lawson, E.J.; White, J.R. Rapid measurement of polymer photo-degradation by FTIR spectrometry of evolved carbon dioxide. Deg. Stab. 2006, 91, 1086–1096, doi:10.1016/j.polymdegradstab.2005.07.011.
- Mu, Z.; Chen, Q.; Zhang, L.; Guan, D.; Li, H. Photodegradation of atmospheric chromophores: Changes in oxidation state and photochemical reactivity. Chem. Phys. 2021, 21, 11581–11591, doi:10.5194/acp-21-11581-2021.
- Wang, C.-N.; Torng, J.-H. Experimental study of the absorption characteristics of some porous fibrous materials. Acoust. 2001, 62, 447–459, doi:10.1016/S0003-682X(00)00043-8.
- Ojeda, T. Freitas, A.; Birck, K.; Dalmolin, E.; Jacques, R.; Bento, F.; Camargo, F. Degradability of linear polyolefins under natural weathering. Degrad. Stab. 2011, 96, 703–707, doi:10.1016/j.polymdegradstab.2010.12.004.
- Sharratt, V.; Hill, C.A.S.; Kint, D.P.R. A study of early colour change due to simulated accelerated sunlight exposure in Scots pine (Pinus sylvestris). Degrad. Stab. 2009, 94, 1589–1594, doi:10.1016/j.polymdegradstab.2009.04.010.
- Bais, A.F.; Mckenzie, R.L. Aucamp, P.J.; Ilyas, M.; Madronich, S.; Tourpali, K. Ozone depletion and climate change: Impacts on UV radiation. Photobiol. Sci. 2015, 14, 19–52, doi:10.1039/c4pp90032d.
- Vitt, R.; Laschewski, G.; Bais, A.F.; Diémoz, H.; Fountoulakis, I.; Siani, A.-M.; Matzarakis, A. UV-Index climatology for Europe based on satellite data. Atmosphere 2020, 11, 727, doi:10.3390/atmos11070727.
- Martins, J.N.; Freire, E.; Hemadipou, H. Applications and market of PVC for piping industry. Polímeros 2009, 19, 58–62, doi:10.1590/S0104-14282009000100014.
- Kumagai, H.; Tashiro, T.; Kobayashi, T. Formation of conjugated carbon bonds on poly (vinyl chloride) films by microwave-discharge oxygen-plasma treatments. Appl. Polym. Sci. 2005, 96, 589–594, doi:10.1002/app.21487.
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314, doi:10.1016/j.wasman.2015.11.041.
- Miskolczi, N.; Bartha, L.; Angyal, A. Pyrolysis of polyvinyl chloride (PVC)-containing mixed plastic wastes for recovery of hydrocarbons. Energy Fuels 2009, 23, 2743–2749, doi:10.1021/ef8011245.
- Braun, D. Recycling of PVC. Polym. Sci. 2002, 27, 2171–2195, doi:10.1016/S0079-6700(02)00036-9.
- Larché, J.-F.; Bussière, P.-O.; Thérias, S.; Gardette, J.-L. Photooxidation of polymers: Relating material properties to chemical changes. Degrad. Stab. 2012, 97, 25–35, doi:10.1016/j.polymdegradstab.2011.10.020.
- Geuskens, G.; Baeyens-Volant, D.; Delaunois, G.; Lu Vinh, Q.; Piret, W.; David, C. Photo-oxidation of polymers–II. The sensitized decomposition of hydroperoxides as the main path for initiation of the photo-oxidation of polystyrene irradiated at 253.7 nm. Polym. J. 1978, 14, 299–303, doi:10.1016/0014-3057(78)90052-6.
- Yaqoob, A.A.; Noor, N.H.M.; Umar, K.; Adnan, R.; Ibrahim, M.N.M.; Rashid, M. Graphene oxide–ZnO nanocomposite: An efficient visible light photocatalyst for degradation of rhodamine B. Nanosci. 2021, 11, 1291–1302, doi:10.1007/s13204-020-01665-8.
- Huang, Z.; Ding, A.; Guo, H.; Lu, G.; Huang, X. Construction of nontoxic polymeric UV-absorber with great resistance to UV-photoaging. Rep. 2016, 6, 25508, doi:10.1038/srep25508.
- Sonnenschein, M.F.; Guillaudeu, S.J.; Landes, B.G.; Wendt, B.L. Comparison of adipate and succinate polymers in thermoplastic polyurethanes. Polymer 2010, 51, 3685–3692, doi:10.1016/j.polymer.2010.06.012.
- Lu, T.; Solis-Ramos, E.; Yi, Y.; Kumosa, M. UV degradation model for polymers and polymer matrix composites. Degrad. Stab. 2018, 154, 203–210, doi:10.1016/j.polymdegradstab.2018.06.004.
- Rabek, J. Polymer Photodegradation: Mechanisms and Experimental Methods; Champan & Hall: London, UK, 1995; pp. 383–391.
- George, G.A. The mechanism of photoprotection of polystyrene film by some ultraviolet absorbers. Appl. Polym. Sci. 1974, 18, 117–124, doi:10.1002/app.1974.070180110.
- Liu, X.; Gao, C.; Sangwan, P.; Yu, L.; Tong, Z. Accelerating the degradation of polyolefins through additives and blending. Appl. Polym. Sci. 2014, 131, 40750, doi:10.1002/app.40750.
- Balakit, A.A.; Ahmed, A.; El-Hiti, G.A.; Smith, K.; Yousif, E. Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly (vinyl chloride). J. Polym. Sci. 2015, 2015, 510390, doi:10.1155/2015/510390.
- Yousif, E.; El-Hiti, G.A.; Haddad, R.; Balakit, A.A. Photochemical stability and photostabilizng efficiency of poly (methyl methacrylate) based on 2-(6-methoxynaphthalen-2-yl) propanoate metal ions complexes. Polymers 2015, 7, 1005–1019, doi:3390/polym7061005.
- Yousif, E.; El-Hiti, G.A.; Hussain, Z.; Altaie, A. Viscoelastic, spectroscopic and microscopic study of the photo irradiation effect on the stability of PVC in presence of sulfamethoxazole Schiff’s bases. Polymers 2015, 7, 2190–2204, doi:3390/polym7111508.
- Yousif, E.; Hasan, A.; El-Hiti, G.A. Spectroscopic, physical and topography of photochemical process of PVC films in the presence of Schiff base metal complexes. Polymers 2016, 8, 204, doi:10.3390/polym8060204.
- Ali, M.M.; El-Hiti, G.A.; Yousif, E. Photostabilizing efficiency of poly (vinyl chloride) in the presence of organotin (IV) complexes as photostabilizers. Molecules 2016, 21, 1151, doi:10.3390/molecules21091151.
- Ali, G.Q.; El-Hiti, G.A.; Tomi, I.H.R.; Haddad, R.; Al-Qaisi, A.J.; Yousif, E. Photostability and performance of polystyrene films containing 1,2,4-triazole-3-thiol ring system Schiff bases. Molecules 2016, 21, 1699, doi:10.3390/molecules21121699.
- Mohammed, R.; El-Hiti, G.A.; Ahmed, A.; Yousif, E. Poly (vinyl chloride) doped by 2-(4-isobutylphenyl) propanoate metal complexes: Enhanced resistance to UV irradiation. J. Sci. Eng. 2017, 42, 4307–4315, doi:10.1007/s13369-016-2323-z.
- Ahmed, D.S.; El-Hiti, G.A.; Hameed, A.S.; Yousif, E.; Ahmed, A. New tetra-Schiff bases as efficient photostabilizers for poly (vinyl chloride). Molecules 2017, 22, 1506, doi:3390/molecules22091506.
- Ali, M.M.; El-Hiti, G.A.; Yousif, E. Investigation of the photodecomposition rate constant of poly (vinyl chloride) films containing organotin (IV) complexes. Al-Nahrain J. Sci. 2017, 20, 18–23. doi:10.22401/JUNS.20.3.04.
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S. Polyphosphates as inhibitors for poly (vinyl chloride) photodegradation. Molecules 2017, 22, 1849, doi:10.3390/molecules22111849.
- Yousif, E.; Haddad, R.; El-Hiti, G.A.; Yusop, R.M. Spectroscopic and photochemical stability of polystyrene films in the presence of metal complexes. Taibah Univ. Sci. 2017, 11, 997–1007, doi:10.1016/j.jtusci.2017.03.002.
- Ghazi, D.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Alotaibi, M.H. The effect of ultraviolet irradiation on the physicochemical properties of poly (vinyl chloride) films containing organotin (IV) complexes as photostabilizers. Molecules 2018, 23, 254, doi:10.3390/molecules23020254.
- Shaalan, N.; Laftah, N.; El-Hiti, G.A.; Alotaibi, M.H.; Muslih, R.; Ahmed, D.S.; Yousif, E. Poly (vinyl chloride) photostabilization in the presence of Schiff bases containing a thiadiazole moiety. Molecules 2018, 23, 913, doi:10.3390/molecules23040913.
- Hashim, H.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S.; Yousif, E. Fabrication of ordered honeycomb porous poly (vinyl chloride) thin film doped with a Schiff base and nickel (II) chloride. Heliyon 2018, 4, e00743, doi:10.1016/j.heliyon.2018.e00743.
- Yousif, E.; Ahmed, D.S.; El-Hiti, G.A.; Alotaibi, M.H.; Hashim, H.; Hameed, A.S.; Ahmed, A. Fabrication of novel ball-like polystyrene films containing Schiff bases microspheres as photostabilizers. Polymers 2018, 10, 1185, doi:10.3390/polym10111185.
- Alotaibi, M.H.; El-Hiti, G.A.; Hashim, H.; Hameed, A.S.; Ahmed, D.S.; Yousif, E. SEM analysis of the tunable honeycomb structure of irradiated poly (vinyl chloride) films doped with polyphosphate. Heliyon 2018, 4, e01013, doi:10.1016/j.heliyon.2018.e01013.
- El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Hamad, B.A.; Ahmed, D.S.; Ahmed, A.; Hashim, H.; Yousif, E. The morphology and performance of poly (vinyl chloride) containing melamine Schiff bases against ultraviolet light. Molecules 2019, 24, 803, doi:10.3390/molecules24040803.
- Alotaibi, M.H.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Hashim, H.; Hameed, A.S.; Ahmed, A. Evaluation of the use of polyphosphates as photostabilizers and in the formation of ball-like polystyrene materials. Polym. Res. 2019, 26, 161, doi:10.1007/s10965-019-1829-y.
- Hadi, A.G.; Yousif, E.; El-Hiti, G.A.; Ahmed, D.S.; Jawad, K.; Alotaibi, M.H.; Hashim, H. Long-term effect of ultraviolet irradiation on poly (vinyl chloride) films containing naproxen diorganotin (IV) complexes. Molecules 2019, 24, 2396, doi:10.3390/molecules24132396.
- Hadi, A.G.; Jawad, K.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Photostabilization of poly (vinyl chloride) by organotin (IV) compounds against photodegradation. Molecules 2019, 24, 3557, doi:10.3390/molecules24193557.
- Ahmed, A.A.; Ahmed, D.S.; El-Hiti, G.A.; Alotaibi, M.H.; Hashim, H.; Yousif, E. SEM morphological analysis of irradiated polystyrene film doped by a Schiff base containing a 1,2,4-triazole ring system. Petrochem. Res. 2019, 9, 169–177, doi:10.1007/s13203-019-00235-6.
- El-Hiti, G.A.; Ahmed, D.S.; Yousif, E.; Alotaibi, M.H.; Star, H.A.; Ahmed, A.A. Influence of polyphosphates on the physicochemical properties of poly (vinyl chloride) after irradiation with ultraviolet light. Polymers 2020, 12, 193, doi:10.3390/polym12010193.
- Mohammed, A.; El-Hiti, G.A.; Yousif, E.; Ahmed, A.A.; Ahmed, D.S.; Alotaibi, M.H. Protection of poly (vinyl chloride) films against photodegradation using various valsartan tin complexes. Polymers 2020, 12, 969, doi:10.3390/polym12040969.
- Ahmed, D.S.; El-Hiti, G.A.; Ibraheem, H.; Alotaibi, M.H.; Abdallh, M.; Ahmed, A.A.; Ismael, M.; Yousif, E. Enhancement of photostabilization of poly (vinyl chloride) doped with sulfadiazine tin complexes. Vinyl Addit. Techn. 2020, 26, 370–379, doi:10.1002/vnl.21752.
- Mahmood, Z.N.; Yousif, E.; Alias, M.; El-Hiti, G.A.; Ahmed, D.S. Synthesis, characterization, properties, and use of new fusidate organotin complexes as additives to inhibit poly (vinyl chloride) photodegradation. Polym. Res. 2020, 27, 267, doi:10.1007/s10965-020-02245-8.
- Majeed, A.; Yousif, E.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S.; Ahmed, A.A. Stabilization of PVC containing captopril tin complexes against degradation upon exposure to ultraviolet light. Vinyl Addit. Techn. 2020, 26, 601–612, doi:10.1002/vnl.21774.
- Salam, B.; El-Hiti, G.A.; Bufaroosha, M.; Ahmed, D.S.; Ahmed, A.; Alotaibi, M.H.; Yousif, E. Tin complexes containing an atenolol moiety as photostabilizers for poly (vinyl chloride). Polymers 2020, 12, 2923, doi:10.3390/polym12122923.
- Omer, R.M.; Al-Tikrity, E.T.B.; Yousif, E.; El-Hiti, G.A.; Ahmed, D.S.; Ahmed, A.A. Spectroscopic and morphological study of irradiated PVC films doped with polyphosphates containing 4,4’-methylenedianiline. J. Appl. Chem. 2020, 93, 1888–1898, doi:10.1134/S1070427220120113.
- Mohamed, S.H.; Hameed, A.S.; El-Hiti, G.A.; Ahmed, D.S.; Kadhom, M.; Baashen, M.A.; Bufaroosha, M.; Ahmed, A.A.; Yousif, E. A process for the synthesis and use of highly aromatic organosilanes as additives for poly (vinyl chloride) films. Processes 2021, 9, 91, doi:10.3390/pr9010091.
- Mousa, O.G.; El-Hiti, G.A.; Baashen, M.A.; Bufaroosha, M.; Ahmed, A.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Synthesis of carvedilol-organotin complexes and their effects on reducing photodegradation of poly (vinyl chloride). Polymers 2021, 13, 500, doi:10.3390/polym13040500.
- Ahmed, A.; El-Hiti, G.A.; Hadi, A.G.; Ahmed, D.S.; Baashen, M.A.; Hashim, H.; Yousif, E. Photostabilization of poly (vinyl chloride) films blended with organotin complexes of mefenamic acid for outdoor applications. Sci. 2021, 11, 2853, doi:10.3390/app11062853.
- Jasem, H.; Hadi, A.G.; El-Hiti, G.A.; Baashen, M.A.; Hashim, H.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Tin-naphthalene sulfonic acid complexes as photostabilizers for poly (vinyl chloride). Molecules 2021, 26, 3629, doi:10.3390/molecules26123629.
- Ghani, H.; Yousif, E.; Ahmed, D.S.; Kariuki, B.M.; El-Hiti, G.A. Tin Complexes of 4-(Benzylideneamino) benzenesulfonamide: Synthesis, structure elucidation and their efficiency as PVC photostabilizers. Polymers 2021, 13, 2434, doi:10.3390/polym13152434.
- Yaseen, A.A.; Al-Tikrity, E.T.B.; Yousif, E.; Ahmed, D.S.; Kariuki, B.M.; El-Hiti, G.A. Effect of ultraviolet irradiation on polystyrene containing cephalexin Schiff bases. Polymers 2021, 13, 2982, doi:10.3390/polym13172982.
- Yaseen, A.A.; Yousif, E.; Al-Tikrity, E.T.B.; El-Hiti, G.A.; Kariuki, B.M.; Ahmed, D.S.; Bufaroosha, M. FTIR, weight, and surface morphology of poly (vinyl chloride) doped with tin complexes containing aromatic and heterocyclic moieties. Polymers 2021, 13, 3264, doi:10.3390/polym13193264.
- Hadi, A.G.; Baqir, S.J.; Ahmed, D.S.; El-Hiti, G.A.; Hashim, H.; Ahmed, A.; Kariuki, B.M.; Yousif, E. Substituted organotin complexes of 4-methoxybenzoic acid for reduction of poly (vinyl chloride) photodegradation. Polymers 2021, 13, 3946, doi:10.3390/polym13223946.
