The identification of homogeneous subgroups of patients with chronic low back pain (CLBP), based on distinct patterns of motor control, could support the tailoring of therapy and improve the effectiveness of rehabilitation. Differences in the patterns of motor control can be identified using outcome measures based on muscle activation or kinematic movement patterns, representing the outcomes of neural structures and processes. The purpose of this review was (1) to assess if there are differences in motor variability between patients with CLBP and pain-free controls, as well as inter-individually among patients with CLBP, during the performance of functional tasks; and (2) to examine the relationship between motor variability and CLBP across time.
- lower back
- chronic pain
- EMG
- kinematics
- motor control
- motor variability
1. Introduction
2. Current Insights
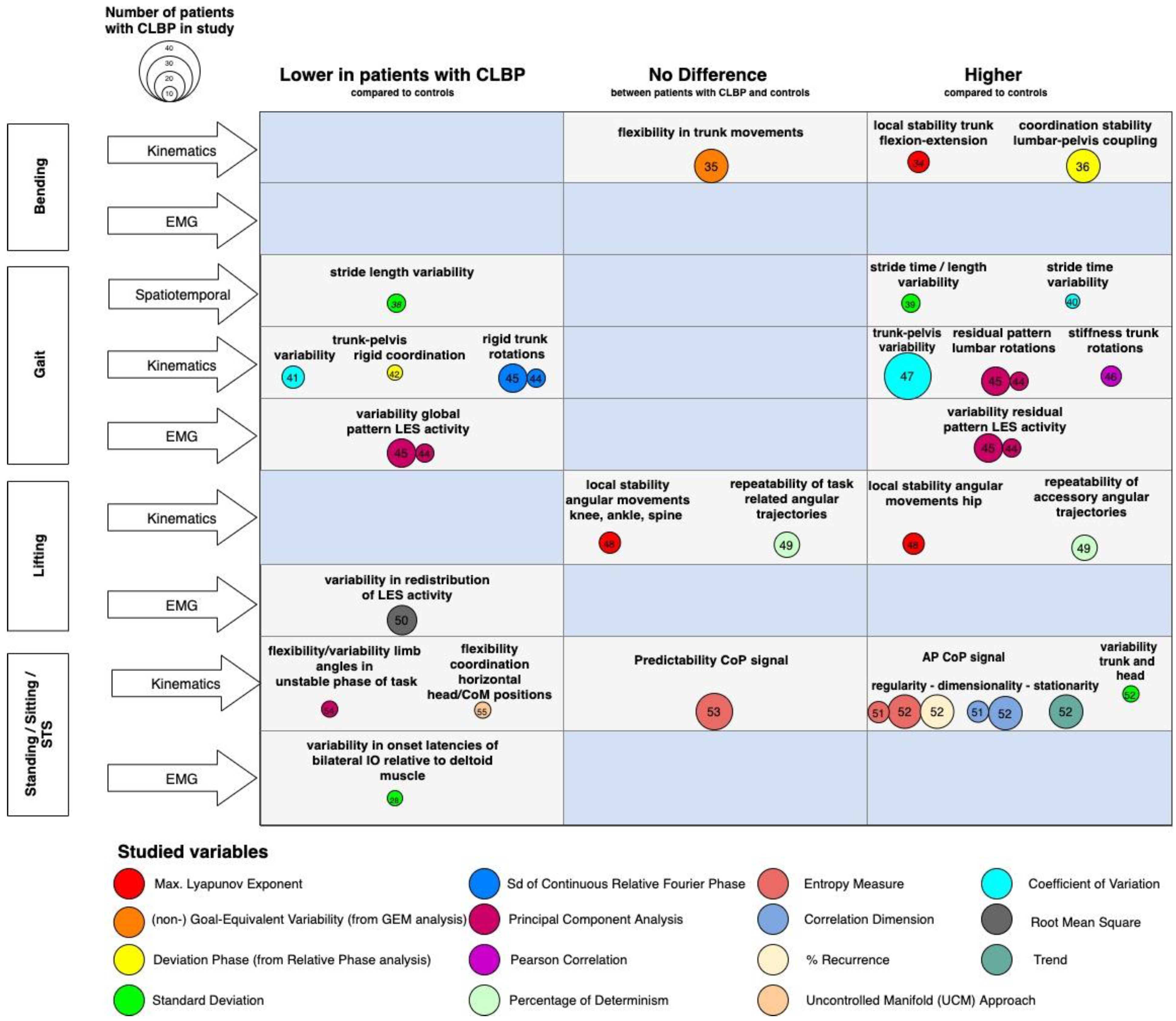
3. Metrics of Motor Variability
4. Heterogeneity in Motor Variability
5. Pain Intensity and Patterns of Motor Variability
6. Changes in Motor Variability in Patients with CLBP: Pathology or Genius Adaptation?
7. Clinical implications
The results of the present woreviewk show that it is not viable to identify homogeneous subgroups of patients with CLBP based on motor variability alone. Longitudinal studies, however, indicate that there is the potential for interventions targeting motor variability. Clinically, the results emphasize the need for the development and study of personalized approaches, and single-subject-like designs, to examine interventions.
References
- Ronai, P.; Sorace, P. Chronic Nonspecific Low Back Pain and Exercise. Strength Cond. J. 2013, 35, 29–32.
- Lambeek, L.C.; van Tulder, M.W.; Swinkels, I.C.; Koppes, L.L.; Anema, J.R.; van Mechelen, W. The Trend in Total Cost of Back Pain in the Netherlands in the Period 2002 to 2007. Spine 2011, 36, 1050–1058.
- Spenkelink, C.D.; Hutten, M.M.R.; Hermens, H.J.; Greitemann, B.O.L. Assessment of activities of daily living with an ambulatory monitoring system: A comparative study in patients with chronic low back pain and nonsymptomatic controls. Clin. Rehabil. 2002, 16, 16–26.
- Oliveira, C.B.; Maher, C.G.; Pinto, R.Z.; Traeger, A.C.; Lin, C.-W.C.; Chenot, J.-F.; van Tulder, M.; Koes, B.W. Clinical practice guidelines for the management of non-specific low back pain in primary care: An updated overview. Eur. Spine J. 2018, 27, 2791–2803.
- Bogduk, N. Management of chronic low back pain. Med. J. Aust. 2004, 180, 79–83.
- Van Tulder, M.W.; Koes, B.; Malmivaara, A. Outcome of non-invasive treatment modalities on back pain: An evidence-based review. Eur. Spine J. 2006, 15, S64–S81.
- O’Sullivan, P. Diagnosis and classification of chronic low back pain disorders: Maladaptive movement and motor control impairments as underlying mechanism. Man. Ther. 2005, 10, 242–255.
- Van Dieën, J.H.; Peter Reeves, N.; Kawchuk, G.; Van Dillen, L.R.; Hodges, P.W. Analysis of motor control in patients with low back pain: A key to personalized care? J. Orthop. Sports Phys. Ther. 2019, 49, 380–388.
- Karayannis, N.V.; Jull, G.A.; Hodges, P.W. Movement-based subgrouping in low back pain: Synergy and divergence in approaches. Physiotherapy 2016, 102, 159–169.
- Foster, N.E.; Hill, J.C.; Hay, E.M. Subgrouping patients with low back pain in primary care: Are we getting any better at it? Man. Ther. 2011, 16, 3–8.
- Mistry, D.; Patel, S.; Hee, S.W.; Stallard, N.; Underwood, M. Evaluating the quality of subgroup analyses in randomized controlled trials of therapist-delivered interventions for nonspecific low back pain: A systematic review. Spine 2014, 39, 618–629.
- Engel, G.L. The clinical application of the biopsychosocial model. Am. J. Psychiatry 1980, 137, 535–544.
- Hush, J.M.; Stanton, T.R.; Siddall, P.; Marcuzzi, A.; Attal, N. Untangling nociceptive, neuropathic and neuroplastic mechanisms underlying the biological domain of back pain. Pain Manag. 2013, 3, 223–236.
- Rabey, M.; Smith, A.; Kent, P.; Beales, D.; Slater, H.; O’Sullivan, P. Chronic low back pain is highly individualised: Patterns of classification across three unidimensional subgrouping analyses. Scand. J. Pain. 2019, 19, 743–753.
- Leboeuf-Yde, C.; Lauritsen, J.M.; Lauritzen, T. Why has the search for causes of low back pain largely been nonconclusive? Spine 1997, 22, 877–881.
- Costa, L.D.C.M.; Koes, B.W.; Pransky, G.; Borkan, J.; Maher, C.G.; Smeets, R.J.E.M. Primary care research priorities in Low Back Pain: An update. Spine 2013, 38, 148–156.
- Tsao, H.; Galea, M.; Hodges, P. Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain 2008, 131, 2161–2171.
- Koch, C.; Hänsel, F. Chronic Non-specific Low Back Pain and Motor Control During Gait. Front. Psychol. 2018, 9, 2236.
- Abboud, J.; Nougarou, F.; Pagé, I.; Cantin, V.; Massicotte, D.; Descarreaux, M. Trunk motor variability in patients with non-specific chronic low back pain. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 114, 2645–2654.
- Van Dieën, J.H.; Flor, H.; Hodges, P.W. Low-Back Pain Patients Learn to Adapt Motor Behavior with Adverse Secondary Con-sequences. Exerc. Sport Sci. Rev. 2017, 45, 223–229.
- van Dieën, J.H.; Prins, M.R.; Bruijn, S.M.; Wu, W.H.; Liang, B.; Lamoth, C.J.C.; Meijer, O.G. Coordination of axial trunk rotations during gait in low back pain. A narrative review. J. Hum. Kinet. 2021, 76, 35–50.
- Stergiou, N.; Decker, L.M. Human movement variability, nonlinear dynamics, and pathology: Is there a connection? Hum. Mov. Sci. 2011, 30, 869–888.
- Scott Kelso, J.A.; Holt, K.G.; Rubin, P.; Kugler, P.N. Patterns of human interlimb coordination emerge from the properties of non-linear, limit cycle oscillatory processes: Theory and data. J. Mot. Behav. 1981, 13, 226–261.
- Daffertshofer, A.; Lamoth, C.J.; Meijer, O.G.; Beek, P.J. PCA in studying coordination and variability: A tutorial. Clin. Biomech. 2004, 19, 415–428.
- Latash, M.L.; Scholz, J.P.; Schöner, G. Motor Control Strategies Revealed in the Structure of Motor Variability. Exerc. Sport Sci. Rev. 2002, 30, 26–31.
- Riley, M.A.; Turvey, M.T. Variability and Determinism in Motor Behavior. J. Mot. Behav. 2002, 34, 99–125.
- Hodges, P.W.; Smeets, R.J. Interaction between pain, movement, and physical activity: Short-term benefits, long-term conse-quences, and targets for treatment. Clin. J. Pain. 2015, 31, 97–107.
- Jacobs, J.V.; Henry, S.M.; Nagle, K.J. People with Chronic Low Back Pain Exhibit Decreased Variability in the Timing of Their Anticipatory Postural Adjustments. Behav. Neurosci. 2009, 123, 455–458.
- Parkhurst, T.M.; Burnett, C.N. Injury and Proprioception in the Lower Back. J. Orthop. Sports Phys. Ther. 1994, 19, 282–295.
- Cholewicki, J.; Breen, A.; Popovich, J.M.; Peter Reeves, N.; Sahrmann, S.A.; Van Dillen, L.R.; Vleeming, A.; Hodges, P.W. Can biome-chanics research lead to more effective treatment of low back pain? A point-counterpoint debate. J. Orthop. Sports Phys. Ther. 2019, 49, 425–436.
- Newell, K.M.; Broderick, M.P.; Deutsch, K.M.; Slifkin, A.B. Task goals and change in dynamical degrees of freedom with motor learning. J. Exp. Psychol. Hum. Percept. Perform. 2003, 29, 379–387.
- Vinet, L.; Zhedanov, A. A “missing” family of classical orthogonal polynomials. J. Phys. A Math. Theor. 2011, 44, 1–25.
- Riccio, G.E.; Newell, K.M.; Corcos, D. Variability and motor control. Am. J. Respir. Crit. Care. Med. 1993, 168, 317–358.
- Stergiou, N.; Harbourne, R.T.; Cavanaugh, J.T. Optimal movement variability: A new theoretical perspective for neurologic physical therapy. J. Neurol. Phys. Ther. 2006, 30, 120–129.
- Newell, K.M.; Mayer-Kress, G.; Liu, Y.-T. Aging, time scales, and sensorimotor variability. Psychol. Aging 2009, 24, 809–818.
- Shafer, D.S. Nonlinear Dynamics and Chaos: With Applications to Physics, Biology, Chemistry, and Engineering (Steven H. Strogatz). SIAM Rev. 1995, 37, 280–281.
- Lamoth, C.J.C.; Meijer, O.G.; Daffertshofer, A.; Wuisman, P.I.J.M.; Beek, P.J. Effects of chronic low back pain on trunk coordination and back muscle activity during walking: Changes in motor control. Eur. Spine J. 2005, 15, 23–40.
- Scholz, J.P.; Schöner, G. Use of the Uncontrolled Manifold (UCM) Approach to Understand Motor Variability, Motor Equivalence, and Self-motion. Adv. Exp. Med. Biol. 2014, 826, 91–100.
- Tajali, S.; Negahban, H.; Shaterzadeh, M.J.; Mehravar, M.; Salehi, R.; Narimani, R.; Parnianpour, M. Multijoint Coordination during Sit-To-Stand Task in People with Non-Specific Chronic Low Back Pain. Biomed. Eng. Appl. Basis Commun. 2013, 25.
- Hamacher, D.D.; Hamacher, D.D.; Schega, L. A cognitive dual task affects gait variability in patients suffering from chronic low back pain. Exp. Brain Res. 2014, 232, 3509–3513.
- Lamoth, C.J.; Stins, J.F.; Pont, M.; Kerckhoff, F.; Beek, P.J. Effects of attention on the control of locomotion in individuals with chronic low back pain. J. Neuroeng. Rehabil. 2008, 5, 13.
- McCaskey, M.A.; Wirth, B.; Schuster-Amft, C.; De Bruin, E.D. Dynamic multi-segmental postural control in patients with chronic non-specific low back pain compared to pain-free controls: A cross-sectional study. PLoS ONE 2018, 13, e0194512.
- Kamper, S.J.; Apeldoorn, A.T.; Chiarotto, A.; Smeets, R.J.E.M.; Ostelo, R.W.J.G.; Guzman, J.; van Tulder, M.W. Multidisciplinary biopsy-chosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2014, 9.
- Woby, S.R.; Watson, P.J.; Roach, N.K.; Urmston, M. Are changes in fear-avoidance beliefs, catastrophizing, and appraisals of control, predictive of changes in chronic low back pain and disability? Eur. J. Pain. 2004, 8, 201–210.
- Loeser, J.D.; Treede, R.-D. The Kyoto protocol of IASP Basic Pain Terminology. Pain 2008, 137, 473–477.
- O’Sullivan, P.; Smith, A.; Beales, D.; Straker, L. Understanding Adolescent Low Back Pain From a Multidimensional Perspective: Implications for Management. J. Orthop. Sports Phys. Ther. 2017, 47, 741–751.
- Srinivasan, D.; Mathiassen, S.E. Motor variability in occupational health and performance. Clin. Biomech. 2012, 27, 979–993.
- Bagheri, R.; Parhampour, B.; Pourahmadi, M.; Fazeli, S.H.; Takamjani, I.E.; Akbari, M.; Dadgoo, M. The Effect of Core Stabilization Exercises on Trunk-Pelvis Three-Dimensional Kinematics During Gait in Non-Specific Chronic Low Back Pain. Spine 2019, 44, 927–936.
- Tsao, H.; Hodges, P.W. Persistence of improvements in postural strategies following motor control training in people with re-current low back pain. J. Electromyogr. Kinesiol. 2008, 18, 559–567.
- McCaskey, M.A.; Wirth, B.; Schuster-Amft, C.; De Bruin, E.D. Postural sensorimotor training versus sham exercise in physiotherapy of patients with chronic non-specific low back pain: An exploratory randomised controlled trial. PLoS ONE 2018, 13, e0193358.
- Hwang, J.A.; Bae, S.H.; Kim, G.D.; Kim, K.Y. The Effects of Sensorimotor Training on Anticipatory Postural Adjustment of the Trunk in Chronic Low Back Pain Patients. J. Phys. Ther. Sci. 2013, 25, 1189–1192.
- Van Dieën, J.H.; Peter Reeves, N.; Kawchuk, G.; Van Dillen, L.R.; Hodges, P.W. Motor control changes in low back pain: Divergence in presentations and mechanisms. J. Orthop. Sports Phys. Ther. 2019, 49, 370–379.
