Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Jorge Domínguez and Version 2 by Rita Xu.
Vermicomposting is the process of organic waste degradation through interactions between earthworms and microbes. A variety of organic wastes can be vermicomposted, producing a nutrient-rich final product that can be used as a soil biofertilizer. Giving the prolific invasive nature of the Australian silver wattle Acacia dealbata Link in Europe, it is important to find alternatives for its sustainable use. However, optimization of vermicomposting needs further comprehension of the fundamental microbial processes.
- 16S rRNA
- earthworms
- metataxonomics
- microbiome
1. Introduction
Vermicomposting is the production of organic amendment via the degradation of organic waste through interactions between earthworms and microbes [1][2][1,2]. While earthworms ingest and process the organic waste (active phase), microbes degrade the organic matter processed by earthworms (maturation phase) during vermicomposting [1]. Additionally, earthworms have a direct impact on the microbial communities since they ingest microbes that can either be digested or released again into the environment [3]. The temporal changes of the microbial community composition and organic matter decay during vermicomposting are an example of heterotrophic ecological succession [4][5][4,5]. The microbial succession is driven by the quantity and quality of the available nutrients in the initial substrate such as organic carbon (e.g., [6]). During vermicomposting, the microbial diversity of the substrate can be modified by the earthworms through gut-associated processes [7][8][7,8]. In addition, the progression of microbial succession is characterized by the replacement of specific groups of bacteria by others, which facilitates substrate utilization and metabolization of the remaining nutrients during the maturation stage (e.g., [6]).
A great variety or organic waste can be effectively vermicomposted including industrial and agricultural wastes [1]. The final product (vermicompost) is rich in microorganisms and nutrients and can be used as a soil biofertilizer [9]. Vermicompost has been shown to promote plant growth due to the presence of growth regulating substances as well as its ability to mitigate or suppress plant diseases (reviewed in [9]). Thus, vermicomposting has the potential to convert plant biomass into high-quality organic biofertilizer [10].
The Australian silver wattle Acacia dealbata Link is one of the most prolific invasive plant species in Europe, threatening native habitats and biodiversity, thus being of great concern [11]. This species is able to invade intensive agricultural areas due to its active dispersal by several animals, water and wind, long-term soil-stored seed banks, and ability to prosper in low-nutrient substrates (reviewed in [11]). Although in Europe some laws prohibit its planting, its presence continues to increase, reducing native biodiversity by competing for resources with the native vegetation [11]. Given the great ecological threat Acacia dealbata represents, it is imperative to find alternatives for its sustainable control and mitigation. A proposed solution is the massive pruning of the species and the use of the generated biomass for bioactive compounds (e.g., [12]) or green manures (e.g., [13]).
2. Changes in Earthworm Density and Microbial Activity during Vermicomposting
Earthworm density significantly increased since the beginning of the trial until day 28, after which it significantly decreased between days 42 and 56 (p < 0.0001, Figure 1, inset). Microbial activity, measured as basal respiration, significantly decreased during vermicomposting until day 28 and then again between days 42 and 56 (p < 0.0001, Figure 1). Additionally, it was possible to see a high correlation between earthworm density and basal respiration (Figure 1).
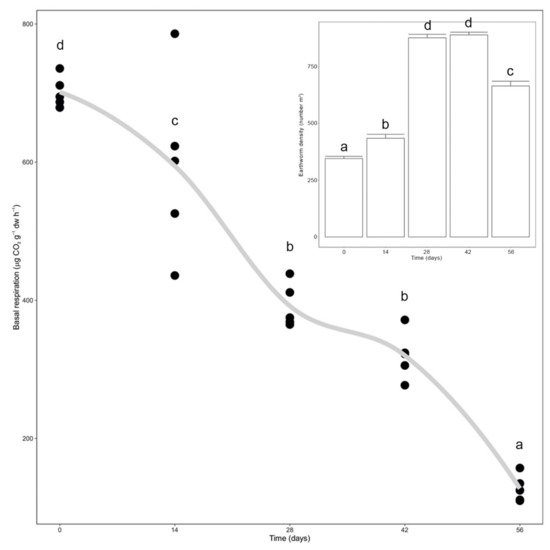

Figure 1. Variation in microbial respiration and earthworm density (inlet) during vermicomposting of the silver wattle. Individual values (n = 5) were plotted for each time point, and the curve was plotted using the “loess” smoothing method in ggplot2. Earthworm biomass values are presented as means ± standard error (n = 5). Letters indicate significant differences between time points.
3. Changes in Bacterial Community Composition during Vermicomposting
The highest number of significantly different taxa was observed between days 0 and 14 (16 phyla, 30 classes, and 177 genera; p < 0.05), except at the ASV level, which was observed between days 14 and 28 (150 ASVs; p < 0.05). Significant differences in bacterial composition decreased up to day 42, increasing again between days 42 and 56.
Initial bacterial community composition (day 0) was dominated by the phylum Proteobacteria, class Gammaproteobacteria, and genus Pseudomonas (Figure 2). The relative abundance of these taxa significantly decreased between days 0 and 14 (p < 0.01; Figure 2). Nevertheless, Proteobacteria continued to dominate the bacterial microbiome along with Bacteroidota, which significantly increased its abundance after 14 days of vermicomposting (p < 0.01; Figure 2). The abundance of sequences belonging to Acidobacteriota, Actinobacteriota, Myxococcota, and Verrucomicrobiota was small but significantly increased during vermicomposting when compared to their relative abundance at day 0 (p < 0.04; Figure 2). Bacteroidia was the dominant class between days 14 and 56, while the relative abundance of the main genera varied (Figure 2).
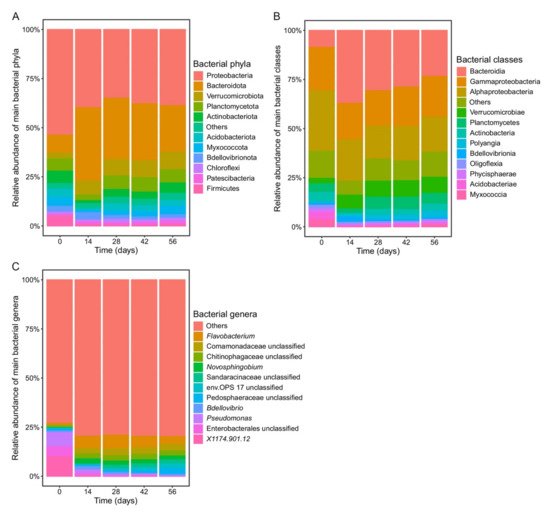

Figure 2. Changes in the bacterial community composition at the phylum (A), class (B), and genus (C) level during vermicomposting of silver wattle. Bars represent the relative abundance of dominant bacterial phyla. Low abundant bacterial taxonomies (relative abundance < 1%) were collapsed into “Others”.
4. Changes in α- and β-Diversity during Vermicomposting
Bacterial communities in fresh silver wattle (day 0) presented low α-diversity for all diversity indices (Figure 3A). After earthworms started vermicomposting, significant increases in α-diversity were observed between days 0 and 14 and days 14 and 28 (p < 0.0001), stabilizing after day 28 (Figure 3A). The only exception to this trend was observed for Faith’s phylogenetic diversity index, with no significant differences observed between days 14 and 28 and days 42 and 56. Significant differences were also observed in phylogenetic and taxonomic β-diversity (p < 0.0001, Figure 3B). Along the first dimension of the principal coordinate analysis plot, the bacterial composition showed significant differences between fresh silver wattle (day 0) and vermicomposted silver wattle (days 14–56) for the weighted unifrac index (Figure 3B). The second dimension reflected the changes in bacterial community composition between all stages of the vermicomposting process (Figure 3B). Samples from days 28, 42, and 56 clustered together, while samples from days 0 and 14 grouped in their own cluster (Figure 3B).
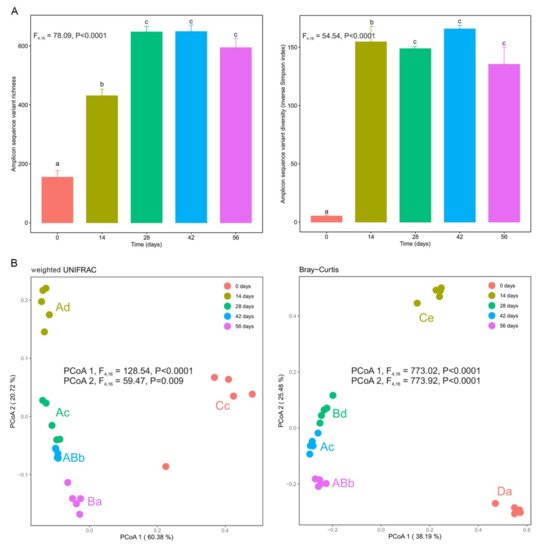

Figure 3. Changes in bacterial α- and β-diversity during vermicomposting of the silver wattle. (A) α-diversity is shown in terms of amplicon sequence variant (ASV) taxonomic richness and diversity (inverse Simpson index). Letters indicate significant differences between time points (Tukey HSD test). (B) β-diversity is shown with principal coordinate analysis (PCoA) of weighted UniFrac and Bray–Curtis distances. Capital and lower case letters indicate significant differences between the time points in PCoA1 and PCoA2 scores, respectively (Tukey HSD test, FDR corrected).
5. Core Microbiome during Vermicomposting
Ten ASVs were identified as the bacterial core microbiome during vermicomposting of silver wattle, being present in all samples from days 14 to 56 (Figure 4). The fresh silver wattle (day 0) was not considered within the core microbiome since these samples were not processed by earthworms. Six of these ASVs belonged to the phylum Proteobacteria and the other four to the phylum Bacteroidota (Figure 4). The phylum Proteobacteria comprised ASVs from the families Comamonadaceae (ASV9) and Moraxellaceae (ASV30), and from the genera Novosphingobium (ASV83), Dokdonella (ASV52), Achromobacter (ASV35), and Methylophylus (ASV116). Among the Bacteroidota present, one ASV belonged to the family Microscillaceae (ASV49) and the remaining to the genera Flavobacterium (ASV3 and ASV6) and Ohtaekwangia (ASV31). The abundance of all 10 ASVs significantly changed between days 0 and 14. Additionally, the abundance of ASV6 (Flavobacterium) differed between days 14 and 28.
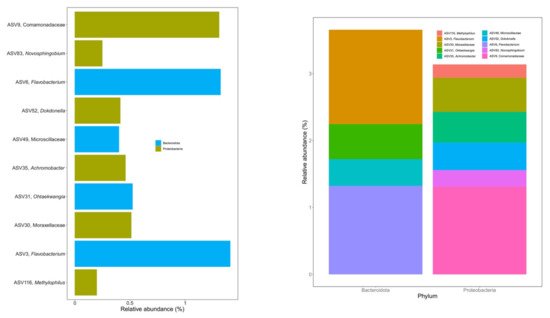

Figure 4. Core microbiome of the silver wattle vermicompost. Initial substrate (day 0) was not considered for determination of the core microbiome. The listed 10 ASVs represent 8.56% of sequences from days 14 to 56 and were found in all samples (n = 20).
6. Functional Diversity during Vermicomposting
Metagenomic predictions using PICRUSt2 showed distinct profiles of certain functional genes such as those involved in cellulose metabolism and nitrification and salicylic acid for different days of vermicomposting (Figure 5). The relative abundance of genes related to cellulose metabolism significantly increased between days 14 and 28 and decreased later between days 42 and 56, with no significant differences between samples from days 0, 14, and 56 (Figure 5A). Genes related to nitrification increased their relative abundance since the beginning up to day 28 of vermicomposting, after which they stabilized (Figure 5B). Genes involved in the synthesis of salicylic acid significantly increased during days 14 and 28, with no significant difference between these two time points, significantly reducing their abundance at day 42 and again at day 56 (Figure 5C).


Figure 5. Changes in gene abundance of PICRUSt-predicted KEGG orthologies implied in cellulose metabolism (A), nitrification (B), and salicylic acid synthesis (C) during vermicomposting of the silver wattle. Values are presented as means ± standard error (n = 5). Above each plot, results from mixed-effects models are shown. Letters indicate significant differences between time points (Tukey HSD test).
