Astaxanthin is a member of the carotenoid family that is found abundantly in marine organisms. It has been reported that astaxanthin functions both as a pigment, and as an antioxidant with superior free radical quenching capacity.
- astaxanthin
- obesity
- mitochondria
- energy metabolisms
- natural antioxidant
- insulin resistance
- AMPK
1. Introduction
1.1. Hidden Bioactivity of Natural Pigments
1.1.1. Nature Is Full of Splendid Color!
1.1.2. Carotenoids
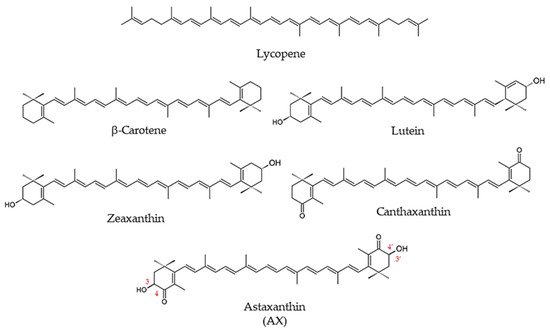
1.1.3. What Is Astaxanthin?
1.2. Biological Activity of Astaxanthin
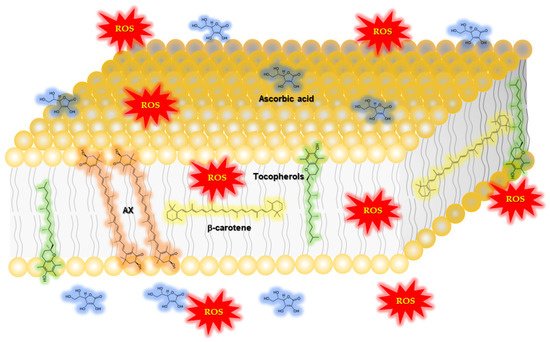
1.2.1. Function of Astaxanthin in Lipid Bilayers: Antioxidant Activity and Impact on Physical Properties
|
Author/Year/Reference |
Study Design |
Subjects |
Dose |
Duration |
Outcome |
|---|---|---|---|---|---|
|
McAllister M.J. et al., |
Randomized, double-blind, placebo-controlled, crossover study |
14 healthy subjects |
0, 6 mg/day |
4 weeks |
Glutathione was ∼7% higher following AX compared with placebo (p < 0.05). No effect on plasma hydrogen peroxide or malondialdehyde (MDA; p > 0.05). Advanced oxidation protein products (AOPP) reduced by ∼28% (N.S.; p = 0.45). |
|
Petyaev I.M., et al., |
Randomized, blinded, four-arm, prospective study |
32 subjects with oxidative stress, 8 subjects taking AX only |
0, 7 mg/day * |
4 weeks |
Reduced serum oxidized LDL by 55.4% after 4 weeks (p < 0.05). Reduced MDA by 52.7% after 4 weeks (p < 0.05). |
|
Chalyk, N. et al., |
Open-label, prospective study |
31 subjects; 18 obese, 8 overweight, 5 healthy weight |
4 mg/day |
92 days |
Plasma MDA decreased with AX by 11.2% on day 15 and by 21.7% on day 29 (N.S.) |
|
Hashimoto H. et al., |
Open-label, prospective study |
35 subjects during cataract surgery |
6 mg/day |
2 weeks |
Superoxide anion scavenging activity (U/mL) 18.2 ± 4.1 at 0 weeks reduced to 19.9 ± 3.6 after 2 weeks of supplementation compared with baseline, p < 0.05. Total hydroperoxides (U CARR) from 1.16 ± 0.18 at 0 weeks reduced to 1.04 ± 0.31 after 2 weeks of supplementation compared with baseline, p < 0.05 |
|
Baralic, I. et al., |
Randomized, double-blind, placebo-controlled, prospective study |
40 healthy subjects (soccer players) |
0, 4 mg/day |
90 days |
Improved prooxidant-antioxidant balance (PAB; p < 0.05) |
|
Baralic I. et al., |
Randomized, double-blind, prospective study |
40 healthy subjects (soccer players) |
0, 4 mg/day |
90 days |
Protected thiol groups against oxidative modification (increase in -SH groups, p < 0.05; improved PON1 activity towards paraoxon and diazoxon, p < 0.05 and p < 0.01, respectively) |
|
Hashimoto, H. et al., |
Open-label, prospective study |
35 cataract patients |
6 mg/day |
2 weeks |
Reduced total hydroperoxides (hydrogen peroxides, lipid peroxides, and peroxides of protein in aqueous humor; p < 0.05), increased superoxide scavenging activity (p< 0.05) |
|
Choi H.D. et al., |
Randomized, two-arm, prospective study |
23 obese and overweight subjects |
5 and 20 mg/day |
3 weeks |
5 mg/day: MDA decreased by 34.6%, isoprostane (ISP) decreased by 64.9%, superoxide dismutase (SOD) increased by 193%, and total antioxidant capacity (TAC) increased by 121% after 3 weeks compared with baseline (p < 0.01). 20 mg/day: MDA decreased by 35.2%, ISP decreased by 64.7%, SOD increased by 194%, and TAC increased by 125% after 3 weeks compared with baseline (p < 0.01). |
|
Choi, H.D. et al., |
Randomized, double-blind, placebo-controlled, prospective study |
27 overweight subjects |
0, 20 mg/day |
12 weeks |
MDA reduced by 17.3% and 29% after 8 and 12 weeks compared with placebo (p < 0.01), isoprostane (ISP) reduced by 40.2% and 52.9% after 8 and 12 weeks compared with placebo (p < 0.01), superoxide dismutase (SOD) increased by 124.8% after 12 weeks compared with placebo (p < 0.01), and total antioxidant capacity (TAC) increased by 130.1% after 12 weeks compared with placebo (p < 0.05) (See Table 3 for other outcomes.) |
|
Hashimoto H. et al., |
Open-label, prospective study |
35 cataract patients |
6 mg/day |
2 weeks |
Reduced total hydroperoxides (hydrogen peroxides, lipid peroxides, and peroxides of protein in aqueous humor; p < 0.05) |
|
Kim, J.H. et al., |
Randomized, Repeated, measured, prospective study |
39 heavy smokers, 39 non-smokers |
0, 5, 20, or 40 mg/day |
3 weeks |
5 mg/day: MDA and ISP significantly lower after 2 and 3 weeks compared with baseline in smokers (p < 0.05). SOD and TAC significant increase after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05) 20 mg/day: MDA and ISP significantly lower after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05). SOD and TAC significant increase after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05). 40 mg/day: MDA and ISP significantly lower after 1, 2, and 3 weeks compared with baseline in smokers (p < 0.05). SOD and TAC significant increase after 2 and 3 weeks compared with baseline in smokers (p < 0.05) |
|
Nakagawa K. et al., |
Randomized, double-blind, placebo-controlled, prospective study |
30 healthy subjects |
0, 6, 12 mg/day |
12 weeks |
6 mg/day: reduction in total phospholipid hydroperoxides (PLOOH) after 12 weeks compared with baseline (p < 0.01) and compared with placebo (p < 0.05). Reduced phosphatidyl-ethanolamine hydroperoxide (PEOOH) after 12 weeks compared with baseline (p < 0.05) and compared with placebo (p < 0.05). 12 mg/day: 48% reduction in total PLOOH after 12 weeks compared with baseline (p < 0.01) and 35% less total PLOOH at 12 weeks compared with the control group (p < 0.05). The 12 mg/day group had 46% less phosphatidylcholine hydroperoxide (PCOOH) at 12 weeks compared with baseline (p < 0.01). |
|
Peng L. et al., |
Randomized, placebo-controlled study |
115 healthy subjects |
0, 40 mg/day |
90 days |
Comparing with the control group, MDA contents in the test group decreased significantly (p < 0.01), and SOD and GSH-Px activities increased significantly (p < 0.01). |
|
Park J.S. et al., |
Randomized, double-blind, placebo-controlled, prospective study |
42 healthy subjects |
2 or 8 mg/day |
8 weeks |
2 mg/day: Concentrations of plasma 8-hydroxy-2′-deoxyguanosine reduced after 4 weeks and 8 weeks compared with placebo (p < 0.05). 8 mg/day: Concentrations of plasma 8-hydroxy-2′-deoxyguanosine reduced after 4 weeks and 8 weeks compared with placebo (p < 0.05) |
|
Iwabayashi M. et al., |
Open-label, prospective study |
35 healthy subjects (with high oxidative stress) |
12 mg/day |
8 weeks |
Increased blood biological antioxidant potential (BAP; +4.6%, p < 0.05) |
|
Yamada T. et al., |
Open-label,prospective study |
6 healthy subjects and 6 Sjoegren’s syndrome subjects |
12 mg/day |
2 weeks |
Reduced protein oxidation (−10%, p < 0.05) |
|
Fassett, R.G. et al., |
Randomized, double-blind, placebo-controlled, prospective study |
58 renal transplant recipients |
0, 12 mg/day |
12 months |
Total plasma F2-isoprostanes reduced by 23.0% in placebo and 29.7% in AX groups (N.S.) |
|
Karppi, J. et al., |
Randomized, double-blind, placebo-controlled, prospective study |
39 healthy subjects |
0, 8 mg/day |
3 months |
Decreased oxidation of fatty acids in healthy men (p < 0.05) |
|
Kim Y.K. et al., |
Open-label, prospective study |
15 healthy postmenopausal women |
0, 2, 8 mg/day |
8 weeks |
Decreased plasma TBARS levels: 2 mg group from 1.42 ± 0.18 to 1.13 ± 0.18 nM/mg (p < 0.05). 8 mg AX group from 1.62 ± 0.14 nM/mg to 1.13 ± 0.12 nM/mg after 8 weeks (p < 0.05). Increased TAS from 0.85 ± 0.42 mM/L to 1.90 ± 0.58 mM/L in the 8 mg group. Urinary 8-isoprostanes excretion did not decrease significantly. (See Table 3 for other outcomes.) |
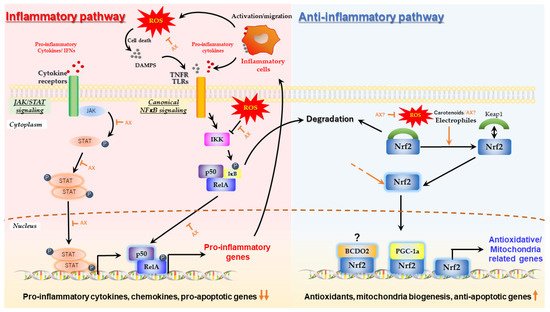
2. Mechanism by Which Astaxanthin Enhances Mitochondrial Energy Metabolism
2.1. Protective Effect of Astaxanthin on Mitochondria; Astaxanthin as a Mitochondrial Antioxidant
2.2. Aggressive Enhancement of Mitochondrial Activity and Metabolism via Gene Expression by Astaxanthin
3. Prospect of Astaxanthin for Human Health Promotion
In rodents such as mice and rats, effective concentrations of AX were probably achieved at the doses used in the study in the targeted organs, and the medications were considered to be effective. Importantly, the doses of AX given to animals in the pharmacological studies presented in this work were quite high. The concentration of AX in the blood of humans and rodents deviates greatly, with the former reaching considerably higher concentrations than the latter [47][95][96][97][98][99][49,108,127,243,244,245]. In humans, although differences in absorption were observed in each clinical trial, this was thought to be due to dietary conditions, formulation, and individual differences. Therefore, it can be confidently expected that the benefits of AX for human subjects can be demonstrated by designing the formulation and administration method. Although they still remain to be improved, we summarized the human clinical studies reported to date on the antioxidant effects of AX (Table 1), as well as its impact on physical activity (Table 2) and cardiovascular, endocrine, and metabolic effects (Table 3). Based on the outcomes presented in Table 1, Table 2 and Table 3, AX can be expected to be especially useful in the prevention of metabolic diseases associated with obesity, T2DM, and sarcopenia, based on the mechanisms described in this work. The effects of AX are only mild, based on the results of clinical studies, and are additive to exercise, so it should be used in combination with standard therapeutic interventions and exercise therapy. Therefore, further research studies are warranted to elucidate the exact mechanism of action in more detail and consider the interaction with the mechanism of medication. Table 2. Human clinical studies of AX on physical performance, endurance and fatigue.|
Author/Year/Reference |
Study Design |
Subjects |
Dose |
Duration |
Outcome |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Outcome | |||||||||||
|
<Subjects: healthy athletes, high daily physical activity> |
|||||||||||
|
Shokri-Mashhadi, N. |
Randomized, double-blind, placebo-controlled, prospective study |
44 patients with type 2 diabetes |
0, 8 mg/day |
8 weeks |
Decrease plasma levels of MDA and IL-6 (p < 0.05) and decrease the expression level of miR-146a, associated with inflammatory markers (fold change: −1/388) (p < 0.05). |
||||||
|
Randomized, double-blind, placebo-controlled, crossover study |
Randomized controlled Open-label, prospective study 12 recreationally trained male cyclists 27.5 ± 5.7 years, VO2peak: 56.5 ± 5.5 mL⋅kg |
26 healthy male −1⋅min−1, subjectsWmax: 346.8 ± 38.4 W |
0, 12 mg/day |
N/A (1 mg AX/100 g salmon) * 7 days |
10 weeks Completion time of the 40-km cycling time trial improved by 1.2 ± 1.7% with AX supplementation, from 70.76 ± 3.93 min in the placebo condition to 69.90 ± 3.78 min in the AX condition (mean improvement time = 51 ± 71 s, p = 0.029, g = 0.21). |
Higher resting oxygen consumption after training in the intervention group only (p < 0.05). Serum carbonylated protein level as an oxidative stress marker tended to be lower immediately after exercise than before exercise in the intervention group only (p = 0.056). (See Table 2. for other outcomes.) Whole body fat oxidation rate was also greater in the AX group between 39–40 km (+0.09 ± 0.13 g⋅min−1, p = 0.044, g = 0.52) and respiratory exchange ratio was lower (−0.03 ± 0.04, p = 0.024, g = 0.60). |
|||||
|
Kato T. et al., | Randomized, double-blind, placebo-controlled, prospective study |
Open-label, prospective study 28 recreational runners (42 ± 8 years) |
16 patients with systolic heart failure 0, 12 mg/day |
12 mg/day * 8 weeks |
3 months Reduced average heart rate at submaximal endurance intensities (aerobic threshold, AeT and anaerobic threshold, AT), but not at higher “peak” intensities. |
||||||
Increased left ventricular ejection fraction (LVEF) from 34.1 ± 8.6% to 38.0 ± 10.0% | ( | p | = 0.031) and 6-min walk distance increased from 393.4 ± 95.9 m to 432.8 ± 93.3 m ( p = 0.023). Significant relationships were observed between percent changes in dROM level and those in LVEF. |
Randomized, double-blind, | |||||||
|
Chan K. et al., | placebo-controlled prospective study |
Randomized controlled Open-label, prospective study 32 well-trained male cyclists 25 ± 5 years, V˙O2 |
54 patients with type 2 diabetes peak = 60 ± 5 mL·kg−1·min−1, Wmax = 5.4 ± 0.5 W·kg−1 |
0, 20 mg/day * |
0, 6, 12 mg/day 4 weeks |
8 weeks N.S; effect on exercise-induced cardiac troponin T release (p = 0.24), changes in antioxidant capacity markers (trolox equivalent antioxidant capacity, uric acid, and malondialdehyde). Markers of inflammation (high-sensitivity C-reactive protein) and exercise-induced skeletal muscle damage (creatine kinase). |
|||||
Increased plasma AX levels and decreased fasting plasma glucose and HbA1c levels. | In 12 mg AX group, reduced in plasma triglyceride, total chol and LDL levels. | Lowered changes in plasma IL-6 and TNF-α levels and plasma vWF level and higher changes in AT-III level. In 12 mg AX group, decreased changes in plasma FVII and PAI-1 levels. |
Res T. et al., |
||||||||
|
Takami M. et al., |
Randomized, double-blind, placebo-controlled, prospective study |
Open-label, prospective study 32 trained male cyclists or triathletes 25 ± 1 years, V˙O2peak = 60 ± 1 mL·kg−1·min−1, Wmax = 395 ± 7 W |
20 healthy young male subjects 0, 20 mg/day |
c.a, 4.5 mg/day * from salmon 4 weeks |
N.S; total plasma antioxidant capacity (p = 0.90) or attenuated malondialdehyde levels (p = 0.63). Whole-body fat oxidation rates during submaximal exercise (from 0.71 +/− 0.04 to 0.68 ± 0.03 g⋅min−1 and from 0.66 ± 0.04 to 0.61 ± 0.05 g⋅min−1 in the placebo and AX groups, respectively; p = 0.73), time trial performance (from 236 ± 9 to 239 ± 7 and from 238 ± 6 to 244 ± 6 W in the placebo and AX groups, respectively; p = 0.63). |
||||||
4 weeks | Higher carbohydrate oxidation during rest in the post-training than that in the pre-training only in the antioxidant group. More decreased levels of serum insulin and HOMA-IR after training were observed in the antioxidant group than in the control group. |
(See Table 2. for other outcomes.) |
|||||||||
Randomized, Double-blind, |
Randomized, placebo-controlled, prospective study |
double-blind, placebo-controlled, prospective study 32 male elite soccer players |
44 participants with type 2 diabetes 0, 4 mg/day |
0, 8 mg/day 90 days |
8 weeks Changes in elevated O2-¯ concentrations after soccer exercise were statistically significant only in the placebo group (exercise × supplementation effect, p < 0.05); TAS values decreased significantly only in the placebo group after exercise (p < 0.01). After intervention, total SH group content increased (21% and 9%, respectively), and the effect of AX was marginally significant (p = 0.08). Basal SOD activity was significantly reduced in both the placebo and AX groups at the end of the study (main training effect, p < 0.01). Post-exercise CK and AST levels were significantly lower in the AX group than in the placebo group (p < 0.05) |
||||||
Increased the serum adiponectin concentration, reduced visceral body fat mass ( | p | < 0.01), serum triglyceride and VLDL chol concentrations, systolic blood pressure, fructosamine concentration ( | p | < 0.05) and marginally reduced the plasma glucose concentration (p = 0.057). |
Earnest C.P. et al., |
Randomized, double-blind, placebo-controlled, | |||||
|
Canas J. A. et al., |
prospective study |
Randomized, double-blind, placebo-controlled, prospective study 14 amateur endurance-trained subjects 18–39 years, V˙O2peak = 52.84 ± 3.5 mL·kg−1·min−1, W |
20 children with simple obesity (BMI > 90%) max = 330 ± 26 W |
0, 4 mg/day |
500 μg/day * (MCS) 28 days |
6 months Improved performance in the 20-km cycling time trial in the AX group (n = 7, −121 s; 95% CI, −185, −53), but not in the placebo group (n = 7, −19 s; 95% CI, −84, 45). AX group significantly increased power output (20 W; 95% CI, 1, 38), whereas the placebo group did not (1.6 W; 95% CI, −17, 20). N.S; carbohydrate, fat oxidation and blood indices indicative of fuel mobilization. |
|||||
Mixed-carotenoid supplementation (MCS) increased β-carotene, total adiponectin, and high-molecular-weight adiponectin in plasma compared with placebo; MCS decreased BMI z-score, waist-to-height ratio, and subcutaneous adipose tissue compared with placebo. AX was used as a part of MCS. | |||||||||||
|
Takemoto M. et al., |
Randomized, placebo-controlled, prospective study |
20 resistance trained male subjects (25.1 ± 1.6 years) |
0, 4 mg/day * |
3 months |
N.S; Muscle soreness, creatine kinase (CK), and muscle performance were measured before and through 96-h post-eccentric exercise |
||||||
] |
Case report |
1 Werner syndrome patient |
12 mg/day * |
6 months |
Improved blood transaminase concentrations before AX intervention and 3 and 6 months after initiation were: AST 40 IU/L, 41 IU/L, and 20 IU/L; ALT 69 IU/L, 62 IU/L, and 34 IU/L; GGT 38 IU/L, 41 IU/L, and 35 IU/L; and cholinesterase 360 IU/L, 366 IU/L, and 331 IU/L, respectively. Liver-to-spleen Hounsfield units on CT were 0.41 before AX initiation, 0.71 at 3 months, and 0.94 at 6 months. No significant changes after AX intervention in hyaluronic acid, a marker of liver fibrosis; high-sensitivity C-reactive protein, a marker of inflammation; and MDA-modified LDL. |
Sawaki K. et al., |
|||||
|
Ni Y. et al., |
Randomized double-blind placebo-controlled, prospective study |
16 healthy adult |
Randomized, single-blind, placebo-controlled, prospective study male subjects |
0, 6 mg/day |
12 NASH patients4 weeks |
12 mg/day |
24 weeksIn the AX group, the serum lactate concentration after 2 min of activity (1200 m run) was significantly lower than that in the control group. |
||||
Improved steatosis ( | p | < 0.05), marginally improved lobular inflammation ( | p = 0.15) and NAFLD activity score (p = 0.08) |
<Subjects: healthy subjects> |
|||||||
|
Choi H.D. et al., |
Randomized, double-blind, placebo-controlled, prospective study |
27 overweight subjects (BMI >25.0 kg/m2) |
0, 20 mg/day |
12 weeks |
Decreased LDL chol and ApoB. (See Table 1. For other outcomes.) |
||||||
|
Yoshida H. et al., |
Randomized controlled |
Randomized, ouble-blind, open-label, prospective study |
placebo-controlled, 26 healthy male subjects |
N/A (1 mg AX/100 g salmon) * |
prospective study 10 weeks |
61 non-obese subjects with fasting serum triglyceride of 120–200 mg/dL and without diabetes and hypertension |
0, 6, 12, 18 mg/day |
12 weeks The skeletal muscle mass was higher after training than before training in both control and intervention groups (p < 0.05). Increased maximal voluntary contraction after training in the intervention group (p < 0.05), but not significantly increased in the control group. (See Table 3 for other outcomes.) |
|||
Multiple comparison: triglycerides were significantly decreased by 12 and 18 mg/day and HDL-cholesterol was significantly increased by 6 and 12 mg. Serum adiponectin was increased by AX (12 and 18 mg/day), and changes in adiponectin were positively correlated with changes in HDL-chol. |
Randomized, double-blind, placebo-controlled, prospective study |
22 healthy subjects |
|||||||||
|
Satoh A. et al., |
Open-label, prospective study |
20 subjects at risk for developing metabolic syndrome (from 127 healthy subjects) |
0, 12 mg/day |
4, (8, 20) mg/day 30 days |
4 weeks. Decreased raise in blood lactate caused by the VO2 Max test; AX group (9.4 ± 3.1 and 13.0 ± 3.1 mmole⋅L−1 in the AX and placebo groups, respectively p < 0.02). Change in oxygen uptake during recovery (−2.02 ± 0.64 and 0.83 ± 0.79% of VO2 Max in the AX and placebo group, respectively, p = 0.001). N.S; anaerobic threshold or VO2 Max. physiological or biochemical differences in the heat tolerance test (HTT) (2 h walk at 40 °C, 40% relative humidity. |
||||||
When subjects who met the diagnostic criteria for metabolic syndrome in Japan (SBP ≥ 130 mmHg, DBP ≥ 85 mmHg, TG ≥ 150 mg/dL, FG ≥ 100 mg/dL) at the start of the study were selected from 4 mg group, significant decreased in SBP( | p | < 0.01). On the other hand, there was no significant decrease in DBP. Reduced TG after treatment (218 mg/dL) than the baseline value (292 mg/dL), marginally reduced fasting glucose after the intervention ( | p | < 0.1). |
Takami M. et al., 2019 [110] | ||||||
|
Uchiyama A. et al., 2008 [126][162] [203] |
Open-label, prospective study |
Open-label, prospective study 20 healthy young male subjects |
c.a, 4.5 mg/day * from salmon |
17 subjects at risk for developing metabolic syndrome4 weeks |
8 mg twice day |
3 months Increased maximum workload by training in both groups (p = 0.009), and increased oxygen consumption during exercise in the antioxidant group only (p = 0.014). There were positive correlations between maximum workload and fat (r = 0.575, p = 0.042) and carbohydrate oxidations (r = 0.520, p = 0.059) in the antioxidant group. (See Table 3 for other outcomes.) |
|||||
Significant decreases plasma HbAlc ( | p | = 0.0433) and TNF-α levels ( | p | = 0.0022) and increase adiponectin concentration (p = 0.0053). N.S: body weight, BMI and waist circumference. |
Imai A. et al., |
Randomized, double-blind, | |||||
placebo-controlled, crossover study |
Randomized, double-blind, placebo-controlled, prospective study 42 healthy subjects |
32 healthy subjects 0, 6 mg/day * |
4 weeks |
0, 6 mg/day Elevated PCOOH levels during mental and physical tasks were attenuated by AX supplementation. Improved recovery from mental fatigue compared with the placebo. No differences were found between AX and the placebo in other secondary outcomes, such as subjective feelings, work efficiency, and autonomic activity. |
|||||||
6 weeks | Synergistic effects of AX intake (12 mg/day, 6 weeks) and aerobic exercise (walking) were studied. AX contributed to reduction of body fat and suppressed the increase in blood lactate level after exercise. |
Hongo N. et al., |
Randomized, | ||||||||
|
Kim Y.K. et al., | double-blind placebo-controlled, prospective study |
Open-label, prospective study 39 healthy subjects |
15 healthy postmenopausal female subjects0, 12 mg/day * |
0, 2, 8 mg/day 12 weeks |
8 weeks Intent-to-treat (ITT) analysis; fatigue after physical and mental stress was significantly lower in the AX group than in the placebo at week 8; the change in POMS Friendliness was significantly higher in the AX group than in the control group at week 8; the rate of change in BAP values at week 12 was not significantly different between the AX and control groups. The rate of change in BAP values at week 12 was not significantly different between the AX group and the control. |
||||||
Increase HDL-chol levels in 2 mg and 8 mg group increased significantly after 8 weeks from 50.6 ± 5.8 to 60.4 ± 7.1 mg/dL, 44.4 ± 10.7 to 49.4 ± 2.7 mg/dL respectively ( | p | < 0.05). In the 2 mg group, triglyceride decreased significantly from 171.6 ± 67.4 mg/dL to 145.8 ± 5.1 mg/dL ( | p | < 0.05). (See Table 1. For other outcomes.) |
Randomized, double-blind, placebo-controlled, prospective study |
40 young healthy subjects (17–19 years) |
0, 4 mg/day |
3 months |
Increased average number of knee bending (squats) increased by 27.05 (from 49.32 to 76.37, AX group) vs. 9.0 (from 46.06 to 55.06, placebo subjects), p = 0.047. |
||
|
Tajima T. et al., |
Randomized, double-blind, placebo-controlled, crossover study |
18 healthy subjects (35.7 ± 4 years) |
0, 5 mg/day |
2 weeks |
Increased in CVRR and HF/TF (Heart rate variability) were significant during exercise at 70% maximum heart rate (HRmax) intensity (p < 0.05). Also, after the AX supplementation, decreased minute ventilation (VE) during exercise at 70% HRmax (p < 0.05). Decreased LDL cholesterol (chol) (p < 0.05) and respiratory quotient after exercise. |
||||||
|
<Subjects: elderly subjects> |
|||||||||||
|
Liu S.Z. et al., |
Randomized, double-blind, placebo-controlled, prospective study |
42 elderly subjects (65–82 years) |
0, 12 mg/day * |
12 weeks |
In endurance training (ET), specific muscular endurance was improved only in the AX group (Pre 353 ± 26 vs. Post 472 ± 41) and submaximal graded exercise test duration was improved in both groups (placebo 40.8 ± 9.1% vs. AX 41.1 ± 6.3%). The increase in fat oxidation at low intensity after ET was greater in AX (placebo 0. 23 ± 0.15 g vs. AX 0.76 ± 0.18 g), and was associated with reduced carbohydrate oxidation and improved exercise efficiency in men, but not in women. |
||||||
|
Liu S.Z. et al., |
Randomized double-blind, placebo-controlled, prospective study |
42 elderly subjects (65–82 years) |
0, 12 mg/day * |
12 weeks |
Administration of AX increased maximal voluntary force (MVC) by 14.4% (± 6.2%, p < 0.02), tibialis anterior muscle size (cross-sectional area, CSA) by 2.7% (± 1.0%, p < 0.01), and specific impulse increased by 11.6% (MVC/CSA, ± 6.0%, p = 0.05), respectively, whereas placebo treatment did not alter these characteristics (MVC, 2.9% ± 5.6%; CSA, 0.6% ± 1.2%; MVC/CSA, 2.4 ± 5.7%; all p > 0.6). |
||||||
|
Fujino H. et al., |
Randomized, double-blind, placebo-controlled, prospective study |
29 community-dwelling healthy elderly subjects (80.9 ± 1.5 years.) |
0, 12 mg/twice a day * |
3 months |
Decrease in d-ROM values with AX group (p < 0.01), but not the placebo group; the AX group had a therapeutic effect on 6-min walking distance compared with the placebo group (p < 0.05). AX group had an increase in distance and number of steps in the 6-min walking test compared with the placebo group. Furthermore, the rate of increase in blood lactate levels after walking was lower in the AX group than in the placebo group (p < 0.01). |
||||||
|
Author/Year/Reference |
Study Design |
Subjects |
Dose |
Duration |
|---|
* In addition to AX, other nutrients such as antioxidants were used in the study.
