Rheumatoid arthritis (RA) and other autoimmune inflammatory diseases are examples of imbalances within the immune system (disrupted homeostasis) that arise from the effects of an accumulation of environmental and habitual insults over a lifetime, combined with genetic predispositions. The Ligand Epitope Antigen Presentation System (LEAPS) therapies are capable of inhibiting ongoing disease progression in animal models. Whereas DMARDs ablate or inhibit specific proinflammatory cytokines or cells and JAK inhibitors (jakinibs) inhibit the receptor activation cascade for expression of proinflammatory cytokines, the LEAPS therapeutic vaccines specifically modulate the ongoing antigen-specific, disease-driving, proinflammatory T memory cell responses. This decreases disease presentation and changes the cytokine conversation to decrease the expression of inflammatory cytokines while increasing the expression of regulatory cytokines.
- immunotherapy
- inflammatory
- anti-inflammatory
- cytokines
- rheumatoid arthritis
1. From Homeostasis to Autoimmunity
2. Post-Translational Modification and Its Role in Autoimmunity
3. Role of Cytokines, Cells, and Their Interplay in Disrupting Immune Homeostasis
In the presence of systemic inflammation, potentially exacerbated by trauma, infection or other inflammatory trigger, autoimmune responses can be initiated against PTM proteins to alter the normal balance of immunity. Figure 1A represents the normal balance in immune pro-inflammatory and anti-inflammatory responses that promote immune homeostasis. The various actors include cells (T and B cells, macrophages, dendritic cells (DC), other blood cells) and pro-inflammatory and anti-inflammatory cytokines that affect the regulation of responses to self (auto)antigen.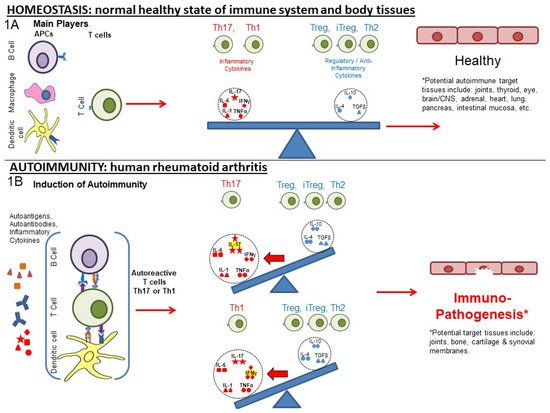
4. Current and Older Therapeutic Approaches for RA
The current therapy for RA consists of either the treatment of symptomatology or the inhibition or ablation of the components of the inflammatory immune response (Table 1). Neither of these approaches address the imbalance of the antigen-specific immune response that is the root cause of the disease. Non-steroidal anti-inflammatory drugs (NSAIDs) are the first line of treatment used to alleviate pain and inflammation. Older ablative treatments with corticosteroid, methotrexate, and similar drugs can inhibit inflammation and are effective for many patients. The newer therapies, as shown in Table 1, are more selective in their inhibition of specific mediators of inflammation and can be organized into three main therapeutic approaches, as shown in Table 1 and Figure 2. Table 1. Approaches to targeting inflammatory cytokines in RA and RA animal models with regard to targets, cytokines, and therapies.| Type | Target | ↓/↑ Modulation | Regulated Immune Component, If Known [References] | Generic and Product name, Regulatory Status | Ref. |
|---|
| Therapeutic Vaccines | Th1 | ↓ | IL-1, IL-17, IFN-γ, TNF-α [3][17][18] | CEL-4000 (preclinical) | [3][17][18] |
| ↑ | Treg (FOXP3+), IL-4, IL-10, TGF-β [3][17][18] | ||||
| Th17 | ↓ | TNF-α, IL-17, IL-6, MCP-1, IL-12p40 [19] | CEL-2000 (preclinical) | [19] | |
| ↑ | IL-12p70, IL-10 [19] | ||||
| DMARDs | TNF-α | ↓ | TNF-α [20] | Adalimumab (Humira®) | [20][21] |
| TNF-α | ↓ | TNF-α [22] | Etanercept (Enbrel®) | [22] | |
| IL-1Ra | ↓ | IL-1 [23] | Anakinra (Kineret®) | [23] | |
| IL-6R msR | ↓ | MCP-1 [21], IL-6 [24] | Tocilizumab (Actemra®) | [21][24] | |
| IL-17 | ↓ | MCP-1 [21], IL-17A [25] | Secukinumab (Cosentyx®) | [21][25] | |
| CD20 | ↓ | B cells as APCs: CD4+IFN-γ+, CD4+IL-17+ [26] | Rituximab (Rituxan®) | [26][27] | |
| Anti-CD6 | ↓ | IL-17 [28], IFN-γ [28][29], IL-6, TNF-α [29] | Itolizumab (Alzumab®) | [28][29][30] | |
| Agonistic Anti-CD137 | ↑ | IFN-γ [31][32], IDO [32] | Utomilumab | [31][32] | |
| Anti-CTLA4 | ↓ | IL-17, IFN-γ [33] | Abatacept (Orencia®) | [33][34][35] | |
| ↑ | IL-35, IFN-β [33] | ||||
| Anti-CD40 | ↓ | IL-6, RANKL [36], TNF-α, NF-κβ, IL-6, ICAM-1, VCAM-1, VEGF [37] | Bi 655064 | [36][37] | |
| CD24 | ↓ | TNF-α, IL-6, MCP-1(CCL2), IL-1β [38] NF-κβ [39] | [38][40][41] | ||
| Jakinibs | JAK3 > JAK1, JAK2 > TYK2 [42] | ↓ | Transcription: IL-2, IL-4, IL-7, IL-9, IL-15, IL-21, IL-6, IL-11, IL-13, IL-25, IL-27, IL-31, IFN-α, IFN-β, IL-10, IL-22, IFN-γ, > EPO, TPO, GH, G-CSF, GM-CSF, Leptin, IL-3, IL-5 > IL-12, IL-23, Type III IFNs [43] in vitro: IL-6 by B cells, [44] IL-2, IL-4, IL-7, IL-15, IL-21, IL-6, and IFN-γ in CD4+ T cells. IL-17 in Th17 cells polarized via IL-23. IL-21 and IL-22 in Th17 [45], IFN-α, IL-6, IFN-γ, IL-2, IL-15, IL-4, GM-CSF [43] MCP-1 [21] IL-17 in CD4+T cells from AS, PSA, RA, and HC [46] in vivo: IL-6 in human [47] |
Tofacitinib (Xeljanz®)FDA approved (2012) | [21][42][43][44][45][46][47][48][49][50] |
| ↑ | in vitro: IL-2 in Th1. IL-17, IL-2 in Th17 cells (polarized via TGF-β1, IL-6) [45] | ||||
| JAK3 > JAK1, TYK2, JAK2 [42] | ↓ | Transcription: IFN-α, IFN-β, IL-10, IL-22, IL-2, IL-4, IL-7, IL-9, IL-15, IL-2, IFN-γ > IL-6, IL-11, IL-13, IL-25, IL-27, IL-31, IL-12, IL-23, Type III IFNs, EPO, TPO, GH, G-CSF, GM-CSF, Leptin, IL-3, IL-5 in vitro: IL-4, IL-13, IFN-γ, TNF-α in PBMC after TCR stimulation, IL-4, IL-13, IFN-γ, TNF-α, IL-17A, GM-CSF in PBMC after IL-2 stimulation [51] |
Peficitinib (Smyraf®) Japan Approved (2019) | [42][51] | |
| JAK2, JAK1 > TYK2 > JAK3 [42] | ↓ | Transcription: IL-6, IL-11, IL-13, IL-25, IL-27, IL-31, IFN-α, IFN-β, IL-10, IL-22, IFN-γ > IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 > IL-12, IL-23, Type III IFNs, EPO, TPO, GH, G-CSF, GM-CSF, Leptin, IL-3, IL-5 [43] in vitro: IL-6 in MoDCs, IFN-α secreted pDCs [44] MCP-1 [21] IL-17 in CD4+ T cells (AS, PSA, RA, and HC) [46] |
Baricitnib (Olumiant®) FDA approved (2018) | [21][42][43][44][46][48][49] | |
| JAK2, JAK1 > TYK2 > JAK3 [42] | ↓ | Transcription: IFN-γ, EPO, TPO, GH, G-CSF, GM-CSF, Leptin, IL-3, IL-5 > IL-6, IL-11, IL-13, IL-25, IL-27, IL-31, IFN-α, IFN-β, IL-10, IL-22, IL-12, IL-23, Type III IFNs > IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 in vitro: IL-10, IFN-γ, IL-6, TNF-α, IL-13 [52] IL-17 in CD4+ (AS, PSA, RA, and HC) [46] in vivo: IFN-γ, IL-12p70, IL-6, G-CSF, IL-10, TNF-α [52] |
Ruxolitinib (Jakafi®) FDA approved (2011) (myelofibrosis) | [42][46][48][52][53] | |
| ↑ | in vitro: IL-2 [53] | ||||
| JAK1 > JAK2 > TYK2 > JAK3 [42] | ↓ | Transcription: IL-6, IL-11, IL-13, IL-25, IL-27, IL-31 > IFN-α, IFN-β, IL-10, IL-22 > IFN-γ, > IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 > EPO, TPO, GH, G-CSF, GM-CSF, Leptin, IL-3, IL-5, IL-12, IL-23, Type III IFNs [43] in vitro: IL-2, IL-4, IFN-αB2, IFN-γ [50] IFN-α, IL-6, IFN-γ, IL-2, IL-15, IL-4 [43] ex vivo: IL-6, GM-CSF [43] in vivo: IFN-γ, IL-6, IL-1β, RANKL, MMP-3, MMP-13, IP10, XCL1, MCP-1, MIP-1b, MCP-3, MCP-5, M-CSF1, MDC, SCF, KC/GRO, IL-1α [50] SAA, IL-6, IL-1β, GM-CSF, TNF-RI, Resistin, TNF-α, MMP-3, YKL40, VEGF, MMP-1, IL-12, IL-2, IFN-γ, IL-13, IL-5, IL-21, IL-23, IL-17A, IL-7, IL-10, CXCL10, CXCL13, MCP-1, VCAM-1, MIP-1a [54] |
Filgotinib (Jyseleca®) EMA & Japan approved (2020) | [42][43][48][50][54] | |
| JAK1 > JAK2 > JAK3 > TYK2 [42] | ↓ | Transcription: IL-6, IL-11, IL-13, IL-25, IL-27, IL-31, IFN-α, IFN-β, IL-10, IL-22, IFN-γ EPO, TPO, GH, G-CSF, GM-CSF, Leptin, IL-3, IL-5 IL-2, IL-4, IL-7, IL-9, IL-15, IL-21 > IL-12, IL-23, Type III IFNs [43] in vitro: IFN-α, IL-6, IFN-γ, IL-2, IL-4, IL-15, G-CSF [43] |
Upadacitinib (Rinvoq®) FDA approved (2019) | [42][43][49] | |
| JAK2 > JAK1 > TYK2 > JAK3 [55] | ↓ | See main text in vitro: VCAM-1, IL-6 [53] |
Fedratinib (Inrebic®) (2019) (myelofibrosis) | [42][53][56][57][58] | |
| ↑ | in vitro: IL-2 [53] |
5. Grouping of the Therapeutic Approaches
The three groups of therapies shown in Table 1 are presented with the immunological target of the treatment, the cytokine(s) that are affected and whether their amount is increased or decreased and examples of specific drugs for that type of treatment. The information in Table 1 is presented as a “heat map” to assist in revealing trends and patterns. Highlighted in red are the five pro-inflammatory cytokines (IL-1(α or β), IL-6, IL-17, IFN-γ, and TNF-α), in Blue are two anti-inflammatory cytokines (IL-4 and IL-10) and in gray are IL-2, since it seems to play a key regulatory role, and IL-12p70, which promotes IFN-γ responses, which can be pro-inflammatory or anti-inflammatory. The Group I therapies, i.e., LEAPS therapeutic vaccines, focus on the cytokine-secreting, antigen-specific T cells, but not individual cytokines per se. These treatments promote a modification of the cytokine conversation that affects both anti-inflammatory cytokines and pro-inflammatory cytokines, focusing upstream on the source of the response, the memory T cell.LEAPS vaccines are peptides that can be designed to elicit an antigen-directed Th1 or Th2/Treg cytokine conversation depending upon the LEAPS immune cell binding ligand peptide that is attached to a disease-related antigenic peptide [3][17][18][19][62]. The J-ICBL activates DCs which produce IL-12 to promote IFN-γ and Th1 cytokine conversations and responses whereas the DerG-ICBL acts on CD4 T cells to promote Th2 and Treg cytokine conversations and responses. By promoting the appropriate antigen-specific cytokine conversations, immunization with J-LEAPS vaccines elicit anti-viral and anti-tumor responses [63] and have the potential to modulate Th17 responses. The DerG-LEAPS vaccines elicit antibody responses [3][62] and have the potential to modulate Th1 responses.
Group II therapies focus on ablations of individual cytokine action by neutralizing the cytokine or blocking its receptor. Neutralizing monoclonal antibodies or receptor antagonists prevent the action of individual acute phase cytokines, TNF-α, IL-1, IL-6 or mediators of Th17 responses, such as IL-23 or IL-17. Alternatively, antibodies to surface differentiation antigens (CDs) are used to eliminate or inhibit specific cells and their functions. Antibody to CD20 lessens the number of B cells to reduce these RA-promoting, antigen-presenting cells. These therapeutic products, monoclonal antibodies, soluble receptor antagonists, agonists, or modified soluble receptors are collectively referred to as Disease-Modifying Antirheumatic Drugs (DMARDs). The first developed and therefore oldest of the group (II) cytokine-focused therapies are the biological response modifiers (BRM), such as neutralizing monoclonal antibodies, solubilized receptors (sR) and modified soluble receptors (msR) (e.g., for IL-2R: MR-IL-2). As monoclonal antibodies, they are highly specific for the antigenic epitopes present on the target molecules [64][65] and act extracellularly. However, it should be noted that some protein subunits are shared between several cytokines, such as IL-12p40 for both IL-12 and IL-23 cytokines, so that multiple cytokines and their consequences may be affected [66][67]. Similarly, monoclonal antibodies directed towards cell surface markers (e.g., CD20) (other than cytokine receptors), may affect several different types of cells expressing that marker [27][65]. The Group II agents are often administered by injection as an intravenous (IV) bolus or infusion over time because of their much larger sizes (e.g., about 150 kDa for monoclonal antibodies). Group III therapies focus on JAK inhibitors (abbreviated to jakinhib-1, -2 or -3, etc.) which target the JAK/STAT transduction of receptor signals, which activates the transcription and production of one or more cytokines, including not only the pro-inflammatory cytokines, but also potentially therapeutic anti-inflammatory cytokines, notably IL-2, IL-4, IL-10, and TGF-β [43][45][47][61][67][68][69][70].6. Comparisons of LEAPS, Monoablative, and Jakinib Therapies
LEAPS peptide therapeutic vaccines are designed to have an immunomodulatory effect on the T cells driving the disease, as illustrated in Figure 2A,B. In so doing, the LEAPS peptides affect the entire cytokine conversation, increasing the expression of some and decreasing other cytokines to return immunobalance, rather than acting on a single cytokine [3][19][17][18][62].
The monoablative therapies (shown in Figure 2C,D) use a neutralizing monoclonal antibody or receptor-antagonist to specifically block the action of one of the disease-associated cytokines after secretion and not its synthesis. The targets for these therapies include IL-1β, IL-6, IL-17A, IL-23, IL-12, and TNF-α and, under certain circumstances, IFN-γ. These monoablation therapies only indirectly affect other pro-inflammatory cytokines and do not upregulate anti-inflammatory cytokines to rebalance the cytokine conversation.

Figure 2. Comparison of cytokine-targeting therapies to treat autoimmune conditions. LEAPS immunomodulating therapy: (3A) CEL-2000 J-LEAPS vaccine: Immunization of diseased animals activates dendritic cells to promote antigen-specific Th1 responses and IL-10 to modulate the disease driving Th17 and inflammatory cytokine responses and provide therapy. (3B) CEL-4000 and related DerG-LEAPS vaccines: CEL-4000 vaccination of diseased animals activates antigen-specific CD4 Th2 and Treg cells to modulate the disease driving Th1, Th17 and inflammatory cytokine responses. Treatment favors a ratio of increased anti-(IL-4, IL-10) vs. pro-(IFN-γ or IL-17) for cytokine secreting CD4 spleen T cells. Monoablation therapy for inflammatory cytokines (DMARDS): (3C) Neutralizing antibody to IL-1, TNFα, or IL-6 (not shown); (3D) Receptor antagonist inhibition of cytokine action: Neutralization or blocking of cytokine receptor by antibody can prevent systemic action of a specific inflammatory cytokine but also affects antimicrobial and other immune responses. Treatment has no effect on anti-inflammatory cytokines. Inhibition of JAK-tyrosine kinase cascade: (3E–3G) Inhibitors of of different JAKs: Small molecular inhibitors of JAK1, JAK2, JAK3 or tyrosine kinase 2 (TYK2) block the signal transmission from associated cytokine receptors to block inflammatory and regulatory responses, depending upon the JAK(s) that are inhibited. These inhibitors downregulate transcription of one or more cytokine gene, as listed in Table 1.
The third group (III) of therapies (Table 1, Figure 2E–G) are the jakinibs, which act by inhibiting specific receptor-associated JAK/STAT tyrosine kinases, ultimately inhibiting the synthesis and secretion of multiple cytokines (multi-ablative therapy) that are activated by the specific JAK cascade. The jakinibs are small-molecule (~300Da) inhibitors acting on the Janus kinases JAK1, JAK2, JAK3 or TYK2 and have the major advantage of being taken orally [42]. The JAK enzymes most often work in pairs as homo- or heterocomplexes activating STAT molecules to create transcriptional activators and promote the expression of groups of cytokines and other genes. JAK activation or inhibition also influences the expression of different cell surface receptors, including CD4, CD80 and CD86 on T, B and other cells and their associated immune responses [44]. The expression of some JAK enzymes is more restricted to certain cell types than others, such as JAK3 for immune system cells such as B, T, and NK cells. This is a therapeutic advantage.
As can be seen in Table 1, there are at least five different jakinibs approved for RA treatment in the USA, Europe, or Japan and several others are under investigation, each unique in terms of the molecule’s binding preference for its particular cognate JAK or JAK-associated molecule. Different manifestations of treatment occur depending on the relative selectivity of binding and whether it is reversible or irreversible. The representative jakinibs are shown as different-colored (red, blue and orange) hexagonal shapes in Figure 2 for the three examples of jakinibs that downregulate inflammatory and anti-inflammatory cytokines. Jakinib A (Figure 2E red hexagon) has activity which is JAK 3>1 and downregulates the expression of IL-6, IL-17, IFN-γ, TNF-α, IL-4, and IL-10. Jakinib B (Figure 2F blue hexagon) is a JAK 2>1 inhibitor downregulating IL-6, IL-17, IFN-γ, TNF-α, and IL-10. Jakinib C (Figure 2G orange hexagon) is a JAK 1>2 inhibitor downregulating IL-1, IL-6, IL-17, IFN-γ, TNF-α, IL-4, and IL-10. It should be noted that a jakinib specific for only JAK2 cannot be used since the inhibition of JAK2/STAT is associated with lethality early in life [42][56].
Although TNF-α, IL-1, and IL-17 are major targets for monoablation therapy, they are not directly affected by the jakinibs. However, they may be indirectly affected by the inhibition of expression of other cytokines that are supposedly not involved in the JAK signaling pathway; for example, IL-17 is affected indirectly by several of these jakinibs [45][46][56]. Similarly, an indirect effect of jakinibs may also promote the expression of some anti-inflammatory cytokines. The combination therapy targeting several cytokines, as possibly seen for the JAK inhibitors, may be effective, although this is still being debated and new clinical studies will be needed [2][71][72][73].
References
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219.
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Prim. 2018, 4, 1–23.
- Rosenthal, K.S.; Mikecz, K.; Steiner, H.L.; Glant, T.T.; Finnegan, A.; Carambula, R.E.; Zimmerman, D.H.; Zimmerman, D.H. Rheumatoid arthritis vaccine therapies: Perspectives and lessons from therapeutic ligand epitope antigen presentation system vaccines for models of rheumatoid arthritis. Expert Rev. Vaccines 2015, 14, 891–908.
- Arleevskaya, M.; Takha, E.; Petrov, S.; Kazarian, G.; Novikov, A.; Larionova, R. Causal risk and protective factors in rheumatoid arthritis: A genetic update. J. Transl. Autoimmun. 2021, 4, 100119.
- Karmakar, U.; Vermeren, S. Crosstalk between B cells and Neutrophils in Rheumatoid Arthritis. Immunology 2021, 164, 689–700.
- Möller, B.; Kollert, F.; Sculean, A.; Villiger, P.M. Infectious Triggers in Periodontitis and the Gut in Rheumatoid Arthritis (RA): A Complex Story About Association and Causality. Front. Immunol. 2020, 11, 1108.
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196.
- Pawelec, T.F.A.L.G.; Cohen, A.K.A.A.; Witkowski, K.H.J.M. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2021.
- Doyle, H.A.; Mamula, M.J. Autoantigenesis: The evolution of protein modifications in autoimmune disease. Curr. Opin. Immunol. 2012, 24, 112–118.
- Mastrangelo, A.; Colasanti, T.; Barbati, C.; Pecani, A.; Sabatinelli, D.; Pendolino, M.; Truglia, S.; Massaro, L.; Mancini, R.; Miranda, F.; et al. The Role of Posttranslational Protein Modifications in Rheumatological Diseases: Focus on Rheumatoid Arthritis. J. Immunol. Res. 2015, 2015, 712490.
- Spinelli, F.R.; Pecani, A.; Conti, F.; Mancini, R.; Alessandri, C.; Valesini, G. Post-translational modifications in rheumatoid arthritis and atherosclerosis: Focus on citrullination and carbamylation. J. Int. Med. Res. 2016, 44, 81–84.
- Burska, A.N.; Hunt, L.; Boissinot, M.; Strollo, R.; Ryan, B.J.; Vital, E.; Nissim, A.; Winyard, P.G.; Emery, P.; Ponchel, F. Autoantibodies to posttranslational modifications in rheumatoid arthritis. Mediators Inflamm. 2014, 2014, 492873.
- Van Venrooij, W.J.; Pruijn, G.J.M. How citrullination invaded rheumatoid arthritis research. Arthritis Res. Ther. 2014, 16, 1–5.
- Wehr, P.; Purvis, H.; Law, S.C.; Thomas, R. Dendritic cells, T cells and their interaction in rheumatoid arthritis. Clin. Exp. Immunol. 2019, 196, 12–27.
- Sakaguchi, S.; Powrie, F.; Ransohoff, R.M. Re-establishing immunological self-tolerance in autoimmune disease. Nat. Med. 2012, 18, 54–58.
- Zeng, H.; Zhang, R.; Jin, B.; Chen, L. Type 1 regulatory T cells: A new mechanism of peripheral immune tolerance. Cell. Mol. Immunol. 2015, 12, 566–571.
- Zimmerman, D.H.; Mikecz, K.; Markovics, A.; Carambula, R.E.; Ciemielewski, J.C.; Toth, D.M.; Glant, T.T.; Rosenthal, K.S. Vaccination by Two DerG LEAPS Conjugates Incorporating Distinct Proteoglycan (PG, Aggrecan) Epitopes Provides Therapy by Different Immune Mechanisms in a Mouse Model of Rheumatoid Arthritis. Vaccines 2021, 9, 448.
- Mikecz, K.; Glant, T.T.; Markovics, A.; Rosenthal, K.S.; Kurko, J.; Carambula, R.E.; Cress, S.; Steiner, H.L.; Zimmerman, D.H. An epitope-specific DerG-PG70 LEAPS vaccine modulates T cell responses and suppresses arthritis progression in two related murine models of rheumatoid arthritis. Vaccine 2017, 35, 4048–4056.
- Zimmerman, D.H.; Taylor, P.; Bendele, A.; Carambula, R.; Duzant, Y.; Lowe, V.; O’Neill, S.P.; Talor, E.; Rosenthal, K.S. CEL-2000: A therapeutic vaccine for rheumatoid arthritis arrests disease development and alters serum cytokine/chemokine patterns in the bovine collagen type II induced arthritis in the DBA mouse model. Int. Immunopharmacol. 2010, 10, 412–421.
- Navarro-Sarabia, F.; Ariza-Ariza, R.; Hernandez-Cruz, B.; Villanueva, I. Adalimumab for treating rheumatoid arthritis. J. Rheumatol. 2006, 33, 1075–1081.
- Nielsen, M.A.; Lomholt, S.; Mellemkjær, A.; Andersen, M.N.; Buckley, C.D.; Kragstrup, T.W. Responses to Cytokine Inhibitors Associated with Cellular Composition in Models of Immune-Mediated Inflammatory Arthritis. ACR Open Rheumatol. 2020, 2, 3–10.
- Chadwick, L.; Zhao, S.; Mysler, E.; Moots, R.J. Review of Biosimilar Trials and Data on Etanercept in Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2018, 20, 1–9.
- Cavalli, G.; Dinarello, C.A. Anakinra therapy for non-cancer inflammatory diseases. Front. Pharmacol. 2018, 9, 1–21.
- Scott, L.J. Tocilizumab: A Review in Rheumatoid Arthritis. Drugs 2017, 77, 1865–1879.
- Frieder, J.; Kivelevitch, D.; Menter, A. Secukinumab: A review of the anti-IL-17A biologic for the treatment of psoriasis. Ther. Adv. Chronic Dis. 2018, 9, 5–21.
- Hamel, K.; Doodes, P.; Cao, Y.; Wang, Y.; Martinson, J.; Dunn, R.; Kehry, M.R.; Farkas, B.; Finnegan, A. Suppression of proteoglycan-induced arthritis by anti-CD20 B Cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J. Immunol. 2008, 180, 4994–5003.
- Shaw, T.; Quan, J.; Totoritis, M.C. B cell therapy for rheumatoid arthritis: The rituximab (anti-CD20) experience. Ann. Rheum. Dis. 2003, 62, 55–59.
- Bughani, U.; Saha, A.; Kuriakose, A.; Nair, R.; Sadashivarao, R.B.; Venkataraman, R.; Patel, S.; Deshchougule, A.T.; Montero, E.; Pai, H.V.; et al. T cell activation and differentiation is modulated by a CD6 domain 1 antibody Itolizumab. PLoS ONE 2017, 12, e0180088.
- Aira, L.E.; Hernández, P.; Prada, D.; Chico, A.; Gómez, J.A.; González, Z.; Fuentes, K.; Viada, C.; Mazorra, Z. Immunological evaluation of rheumatoid arthritis patients treated with itolizumab. MAbs 2016, 8, 187–195.
- Rodríguez, P.C.; Prada, D.M.; Moreno, E.; Aira, L.E.; Molinero, C.; López, A.M.; Gómez, J.A.; Hernández, I.M.; Martínez, J.P.; Reyes, Y.; et al. The anti-CD6 antibody itolizumab provides clinical benefit without lymphopenia in rheumatoid arthritis patients: Results from a 6-month, open-label Phase I clinical trial. Clin. Exp. Immunol. 2018, 191, 229–239.
- Shuford, W.W.; Klussman, K.; Tritchler, D.D.; Loo, D.T.; Chalupny, J.; Siadak, A.W.; Brown, T.J.; Emswiler, J.; Raecho, H.; Larsen, C.P.; et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J. Exp. Med. 1997, 186, 47–55.
- Dharmadhikari, B.; Wu, M.; Abdullah, N.S.; Rajendran, S.; Ishak, N.D.; Nickles, E.; Harfuddin, Z.; Schwarz, H. CD137 and CD137L signals are main drivers of type 1, cell-mediated immune responses. Oncoimmunology 2016, 5, e1113367.
- Alenazy, M.F.; Saheb Sharif-Askari, F.; Omair, M.A.; El-Wetidy, M.S.; Omair, M.A.; Mitwalli, H.; Al-Muhsen, S.; Al-Masri, A.; Hamid, Q.; Halwani, R. Abatacept enhances blood regulatory B cells of rheumatoid arthritis patients to a level that associates with disease remittance. Sci. Rep. 2021, 11, 1–9.
- Cutolo, M.; Sulli, A.; Paolino, S.; Pizzorni, C. CTLA-4 blockade in the treatment of rheumatoid arthritis: An update. Expert Rev. Clin. Immunol. 2016, 12, 417–425.
- Kawashiri, S.; Endo, Y.; Nishino, A.; Okamoto, M.; Tsuji, S.; Takatani, A.; Shimizu, T.; Sumiyoshi, R.; Koga, T.; Iwamoto, N.; et al. Association between serum bone biomarker levels and therapeutic response to abatacept in patients with rheumatoid arthritis (RA): A multicenter, prospective, and observational RA ultrasound cohort study in Japan. BMC Musculoskelet. Disord. 2021, 22, 506.
- Visvanathan, S.; Daniluk, S.; Ptaszyński, R.; Müller-Ladner, U.; Ramanujam, M.; Rosenstock, B.; Eleftheraki, A.G.; Vinisko, R.; Petříková, A.; Kellner, H.; et al. Effects of BI 655064, an antagonistic anti-CD40 antibody, on clinical and biomarker variables in patients with active rheumatoid arthritis: A randomised, double-blind, placebo-controlled, phase IIa study. Ann. Rheum. Dis. 2019, 78, 754–760.
- Lai, J.-H.; Luo, S.-F.; Ho, L.-J. Targeting the CD40-CD154 Signaling Pathway for Treatment of Autoimmune Arthritis. Cells 2019, 8, 927.
- Zheng, X.; Wu, W.; Liu, Y.; Zheng, P. US Patent Application: Methods of Use of Soluble CD24 for Therapy of Rheumatoid Arthritis. U.S. 2013/0136739 A1, 28 April 2011.
- Chen, G.-Y.; Tang, J.; Zheng, P.; Liu, Y. CD24 and Siglec-10 Selectively Repress Tissue Damage-Induced Immune Responses. Science 2009, 323, 1722–1725.
- Lee, J.; Smeriglio, P.; Dragoo, J.; Maloney, W.J.; Bhutani, N. CD24 enrichment protects while its loss increases susceptibility of juvenile chondrocytes towards inflammation. Arthritis Res. Ther. 2016, 18, 292.
- Salomon, S.; Guignant, C.; Morel, P.; Flahaut, G.; Brault, C.; Gourguechon, C.; Fardellone, P.; Marolleau, J.-P.; Gubler, B.; Goëb, V. Th17 and CD24hiCD27+ regulatory B lymphocytes are biomarkers of response to biologics in rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 33.
- Virtanen, A.T.; Haikarainen, T.; Raivola, J.; Silvennoinen, O. Selective JAKinibs: Prospects in Inflammatory and Autoimmune Diseases. BioDrugs 2019, 33, 15–32.
- Traves, P.G.; Murray, B.; Campigotto, F.; Galien, R.; Meng, A.; Di Paolo, J.A. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann. Rheum. Dis. 2021, 80, 865–875.
- Kubo, S.; Nakayamada, S.; Sakata, K.; Kitanaga, Y.; Ma, X.; Lee, S.; Ishii, A.; Yamagata, K.; Nakano, K.; Tanaka, Y. Janus kinase inhibitor baricitinib modulates human innate and adaptive immune system. Front. Immunol. 2018, 9, 1510.
- Ghoreschi, K.; Jesson, M.I.; Li, X.; Lee, J.L.; Ghosh, S.; Alsup, J.W.; Warner, J.D.; Tanaka, M.; Steward-Tharp, S.M.; Gadina, M.; et al. Modulation of Innate and Adaptive Immune Responses by Tofacitinib (CP-690,550). J. Immunol. 2011, 186, 4234–4243.
- Hammitzsch, A.; Chen, L.; de Wit, J.; Al-Mossawi, M.H.; Ridley, A.; Sekine, T.; Simone, D.; Doig, K.; Skapenko, A.; Bowness, P. Inhibiting ex-vivo Th17 responses in Ankylosing Spondylitis by targeting Janus kinases. Sci. Rep. 2018, 8, 1–8.
- Hodge, J.A.; Kawabata, T.T.; Krishnaswami, S.; Clark, J.D.; Telliez, J.B.; Dowty, M.E.; Menon, S.; Lamba, M.; Zwillich, S. The mechanism of action of tofacitinib—An oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin. Exp. Rheumatol. 2016, 34, 318–328.
- O’Shea, J.J.; Holland, S.M.; Staudt, L.M. JAKs and STATs in immunity, immunodeficiency, and cancer. N. Engl. J. Med. 2013, 368, 161–170.
- Harigai, M.; Honda, S. Selectivity of Janus Kinase Inhibitors in Rheumatoid Arthritis and Other Immune-Mediated Inflammatory Diseases: Is Expectation the Root of All Headache? Drugs 2020, 80, 1183–1201.
- Van Rompaey, L.; Galien, R.; van der Aar, E.M.; Clement-Lacroix, P.; Nelles, L.; Smets, B.; Lepescheux, L.; Christophe, T.; Conrath, K.; Vandeghinste, N.; et al. Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J. Immunol. 2013, 191, 3568–3577.
- Kitanaga, Y.; Imamura, E.; Nakahara, Y.; Fukahori, H.; Fujii, Y.; Kubo, S.; Nakayamada, S.; Tanaka, Y. In vitro pharmacological effects of peficitinib on lymphocyte activation: A potential treatment for systemic sclerosis with JAK inhibitors. Rheumatology 2020, 59, 1957–1968.
- Huarte, E.; Peel, M.T.; Verbist, K.; Fay, B.L.; Bassett, R.; Albeituni, S.; Nichols, K.E.; Smith, P.A. Ruxolitinib, a JAK1/2 Inhibitor, Ameliorates Cytokine Storm in Experimental Models of Hyperin fl ammation Syndrome. Front. Pharmacol. 2021, 12, 650295.
- Singer, J.W.; Al-Fayoumi, S.; Taylor, J.; Velichko, S.; O’Mahony, A. Comparative phenotypic profiling of the JAK2 inhibitors ruxolitinib, fedratinib, momelotinib, and pacritinib reveals distinct mechanistic signatures. PLoS ONE 2019, 14, e0222944.
- Tarrant, J.M.; Galien, R.; Li, W.; Goyal, L.; Pan, Y.; Hawtin, R.; Zhang, W.; Van der Aa, A.; Taylor, P.C. Filgotinib, a JAK1 Inhibitor, Modulates Disease-Related Biomarkers in Rheumatoid Arthritis: Results from Two Randomized, Controlled Phase 2b Trials. Rheumatol. Ther. 2020, 7, 173–190.
- Zhou, T.; Georgeon, S.; Moser, R.; Moore, D.J.; Caflisch, A.; Hantschel, O. Specificity and mechanism-of-action of the JAK2 tyrosine kinase inhibitors ruxolitinib and SAR302503 (TG101348). Leukemia 2014, 28, 404–407.
- Lin, C.M.; Cooles, F.A.; Isaacs, J.D. Basic Mechanisms of JAK Inhibition. Mediterr. J. Rheumatol. 2020, 31, 100.
- Pardanani, A.; Harrison, C.; Cortes, J.E.; Cervantes, F.; Mesa, R.A.; Milligan, D.; Masszi, T.; Mishchenko, E.; Jourdan, E.; Vannucchi, A.M.; et al. Safety and Efficacy of Fedratinib in Patients With Primary or Secondary Myelofibrosis. JAMA Oncol. 2015, 1, 643.
- Blair, H.A. Fedratinib: First Approval. Drugs 2019, 79, 1719–1725.
- Zarrin, A.A.; Bao, K.; Lupardus, P.; Vucic, D. Kinase inhibition in autoimmunity and inflammation. Nat. Rev. Drug Discov. 2021, 20, 39–63.
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018, 27, 1984–2009.
- Moura, R.A.; Fonseca, J.E. JAK Inhibitors and Modulation of B Cell Immune Responses in Rheumatoid Arthritis. Front. Med. 2021, 7, 1105.
- Markovics, A.; Zimmerman, D.H.; Mikecz, K.; Rosenthal, K.S. Suppression of Arthritis by Immunomodulatory LEAPS Peptide Vaccines in Animal Models of Rheumatoid Arthritis. Int. J. Drug Dev. Res. 2021, 13, 9502.
- Ken S. Rosenthal; Daniel H. Zimmerman; J-LEAPS vaccines elicit antigen specific Th1 responses by promoting maturation of type 1 dendritic cells (DC1). ADaniel H. Zimmerman; Roy E. Carambula; Jason Ciemielewski; Ken S. Rosenthal; Lessons from next generation influenza vaccines for inflammatory disease therapies. IMS Allntergy andnational Immunopharmacology 2016, 1, 89-100, 10.3934/allergy.2017.2.89.9, 74, 105729-105729, 10.1016/j.intimp.2019.105729.
- Burmester, G.R.; Feist, E.; Dörner, T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat. Rev. Rheumatol. 2013, 10, 77–88.
- Lipman, N.S.; Jackson, L.R.; Trudel, L.J.; Weis-Garcia, F. Monoclonal versus polyclonal antibodies: Distinguishing characteristics, applications, and information resources. ILAR J. 2005, 46, 258–267.
- Leonard, W.J.; Lin, J.X.; O’Shea, J.J. The γ c Family of Cytokines: Basic Biology to Therapeutic Ramifications. Immunity 2019, 50, 832–850.
- O’Shea, J.J.; Plenge, R. JAK and STAT Signaling Molecules in Immunoregulation and Immune-Mediated Disease. Immunity 2012, 36, 542–550.
- O’shea, J.J.; Kontzias, A.; Yamaoka, K.; Tanaka, Y.; Laurence, A. Janus kinase Inhibitors in autoimmune diseases. Ann. Rheum. Dis. 2013, 72, ii111–ii115.
- Schwartz, D.M.; Bonelli, M.; Gadina, M.; O’shea, J.J. Type I II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat. Rev. Rheumatol. 2016, 12, 25–36.
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. Jak inhibition as a therapetuic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 17, 843–862.
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; Van Vollenhoven, R.F.; De Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, S685–S699.
- Chatzidionysiou, K. Beyond Methotrexate and Biologics in RA—Efficacy of JAK Inhibitors and their Place in the Current Treatment Armamentarium. Mediterr. J. Rheumatol. 2020, 31, 120–128.
- Kotyla, P.J. Are Janus Kinase Inhibitors Superior over Classic Biologic Agents in RA Patients? Biomed Res. Int. 2018, 2018, 7492904.
- Adrienn Markovics; Ken S. Rosenthal; Katalin Mikecz; Roy E. Carambula; Jason C. Ciemielewski; Daniel H. Zimmerman; Restoring the Balance between Pro-Inflammatory and Anti-Inflammatory Cytokines in the Treatment of Rheumatoid Arthritis: New Insights from Animal Models. Biomedicines 2021, 10, 44, 10.3390/biomedicines10010044.
