Studies are divided according to the polymer matrix and the agents the nanoparticles carry. Some nanoparticles have the capability to polarize macrophages themselves without reprogramming agents, and the effects are varied for different modifications and residues. Ann-Kathrin Fuchs et al. found that both carboxyl-modified polystyrene nanoparticles and amino-modified ones succeeded in suppressing macrophages from polarizing towards M2 by down regulating the expression of CD200R, CD163, as well as IL-10, without affecting the M1 markers
[1][45]. Yen-Jang Huang’s group discovered that hydrophilic polyurethane nanoparticles themselves had surface-dependent immunosuppressive properties, preventing macrophages from M1 polarization by decreasing the production of TNF-α and IL-1β
[2][46]. Carboxyl-based nanoparticles were more suppressive than the amino-modified ones
[2][46]. The two studies suggested that the effect of nanoparticles on macrophage polarization does not only depend on functional groups but also on other properties, and the nanomedicines should be estimated as a whole. Recently, membrane-coating technology has been used widely in the biomedical field
[3][4][47,48], and some cellular membranes could be special agents to influence macrophage polarization. For example, cellular membranes of natural killer cells (NKs) and THP1 macrophages were coated with poly(lactic-co-glycolic acid) (PLGA) nanoparticles, resulting in ten times higher IL-6 than that in the control group
[5][49]. Likewise, macrophage membranes were also used to coat PLGA, which delivers iron oxide and TLR agonist R837 to potentiate immunotherapy
[6][50]. C.G. Da Silvaa’s group used PLGA as a biodegradable matrix core to simultaneously deliver R848, poly (I:C) and MIP3α, leading to the inhibition of TC-1 growth in pre-clinical experiments
[7][51].
TLR agonists are significant in macrophage repolarization. Christopher B. Rodell et al. showed that the TLR7/8 agonist, R848, was one of the most powerful molecules for polarizing macrophages in the M1 direction in vitro among 38 immunomodulatory agents reported in the literature
[8][24]. β-cyclodextrin nanoparticles encapsulated with R848 succeeded in regulating TAMs and increased the growth inhibition efficacy of cancer cells in various models together with anti-PD-1 therapy
[8][24]. A lignin nanoparticle was also a candidate to carry R848 and targeted CD206-expressing M2 with specific peptides modified on the surface
[9][52]. In vitro, the nanomedicine successfully promoted M1 marker TNF-α almost twenty times more than the control and reduced the tumor burden in mice
[9][52]. In another group, acetylated chondroitin sulfate protoporphyrin polymer was developed to deliver R837
[10][53]. Together with the other polymeric micelle loading with Dox, it suppressed 4T1 growth in mice
[10][53]. The TLR9 agonist CpG has also been carried to modulate macrophages. Jutaek Nam’s lab
[11][54] used cationic polyethyleneimine (PEI) to absorb CpG and neoantigen peptides to form a polyplex nano-vaccine. In draining lymph nodes (dLNs) of vaccinated mice, the amount of CD86+ M1 increased, and CD206+ M2 decreased
[11][54], showing the capacity of nanovaccines to regulate macrophages. Moreover, TLR agonists and other agents also show great potential for TAM polarization. Plasmid DNA and messenger RNA (mRNA) are two therapeutic agent forms of genetic materials and are also leveraged to edit M1 regulators. For example, a novel lipid-coating polymer termed PQDEA was developed to form polyplexes with IL-12 plasmid and inhibited three tumor models in mice with only four doses
[12][55]. Therefore, to further prompt macrophage repolarization, F. Zhang et al. discovered that polymers coated with M1-polarization-associated mRNA and modified with di-mannose could target the CD206 receptor of macrophages, increase M1 macrophages and suppress three tumor models (
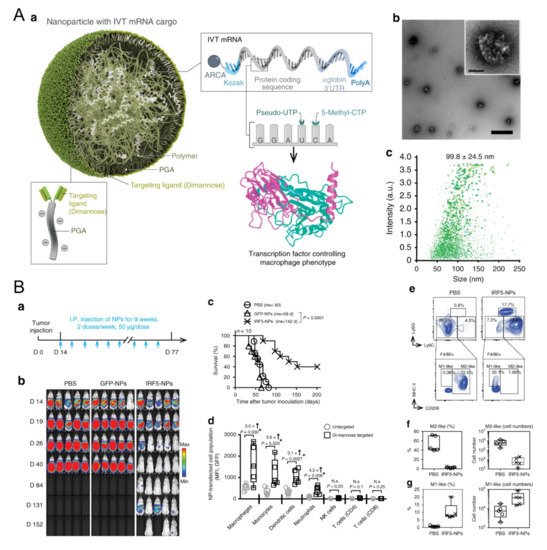
Figure 1)
[13][56]. The M1-associated mRNA coded transcription factors IRF5 and IKKβ, which are downstream proteins in the IFN I pathway, thus promoting M1 skew
[13][56]. Yudong Song et al. also modified their polymer with mannose to carry two short interfering RNAs (siRNAs), which block VEGF and PIGF. As these two factors mediate M2 polarization and monocyte recruitments, blocking them resulted in promoting IL-12 and IFN-γ in TME
[14][57]. HA and miR125b are effective in promoting M1 polarization. HA-PEI nanoparticles loaded with miR125b were fabricated and succeeded in promoting TAM polarization towards the M1 type after being intraperitoneally injected
[15][58]. Another article pointed out that the N-(2-hydroxypropyl) methacrylamide (HPMA)-copolymer nanocarrier could also target CD11b+ TAMs and regulate the TME in situ by inducing M1 polarization
[16][59]. Some biophosphonates, such as zoledronic acid (ZOL), were also used to mediate TAM repolarization, and a pH-sensitive dendritic poly-lysine nanoparticle loaded with ZOL could release it once inside the TME
[17][60]. For the interference of key chemokines, a shrinkable polymer carrying BLZ-945, a CSF1R inhibitor, to regulate TAM succeeded in reducing CD206+ M2 from 40% to 15%, while IL-12 and IFN-γ in TME increased to three times that of the control
[18][61].
Figure 1. Polymeric nanoparticles loaded with mRNA for macrophage repolarization. (
A) Characteristics of the polymeric nanocarriers: (
a) The components of the polymeric nanoparticles (termed IRF5-NPs). (
b) Transmission electron microscopy of the nanoparticles. (
c) Size distribution of the nanocarriers. Reproduced with permission
[13][56]. Copyright 2019 Nature Publishing Group. (
B) IRF5-NPs prolong the survival time of mice with ovarian cancer and reprogram macrophages in vivo. (
a) Treatment schedule. (
b) Tumor growth after intraperitoneal administration of IRF5-NPs. (
c) The survival curves of tumor-bearing mice. (
d) Quantitation of transfection rates in various immune cells using flow cytometry. (
e–
g) The reprogramming effect of IRF5-NPs on peritoneal macrophages in tumor-bearing mice. Reproduced with permission
[13][56]. Copyright 2019 Nature Publishing Group.