Despite advances in antimicrobial therapy and even the advent of some effective vaccines, Pseudomonas aeruginosa (P. aeruginosa) remains a significant cause of infectious disease, primarily due to antibiotic resistance. Although P. aeruginosa is commonly treatable with readily available therapeutics, these therapies are not always efficacious, particularly for certain classes of patients (e.g., cystic fibrosis (CF)) and for drug-resistant strains. Combinations of monoclonal antibodies against different targets and epitopes have demonstrated synergistic efficacy with each other as well as in combination with antimicrobial agents typically used to treat these infections. Such a strategy has reduced the ability of infectious agents to develop resistance. This entreviewy highlights potential targets secreted by by P. aeruginosa that future polyclonal antibodies may directed against in order to develop more efficacious treatments against these infections.
- polyclonal antibodies
- antibiotic resistance
- antibiotics
- Pseudomonas aeruginosa
1. Introduction
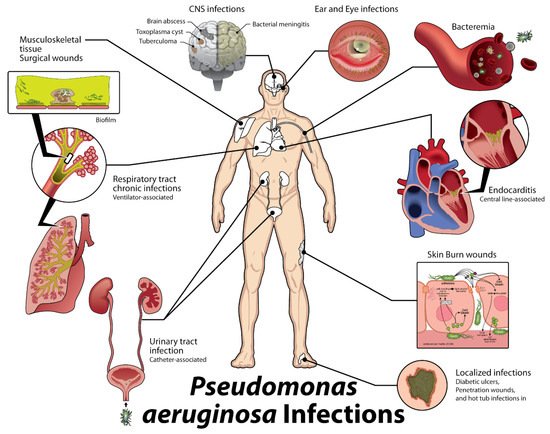
2. Host Immune Response
3. Description of Targets
Taking inspiration from the immune response and in the context of P. aeruginosa’s life cycle and its antibiotic resistance mechanisms, several potential targets secreted by P. aeruginosa were identified. These targets, outlined in Table 1, produce a wide variety of effects in hosts and the bacteria, contributing to the pathogenesis of the entire spectrum of infections caused by this organism.| Location or Class | Examples | Activity/Effects on Host | ||||||
|---|---|---|---|---|---|---|---|---|
| Cell surface | Alginate | Antiphagocytic, resists opsonic killing | ||||||
| Lipopolysaccharide | Endotoxic, antiphagocytic, avoids preformed antibody to previously encountered O antigens | |||||||
| Pili (produced by type IV secretion) | Twitching motility, biofilm formation, adherence to host tissues | |||||||
| Flagella | Motility, biofilm formation, adherence to host tissues and mucin components | |||||||
| Injection of type III secretion factors | PcrG, PcrV, PcrH, PopB, and PopD proteins form injection bridge for type III effectors | |||||||
| Outer membrane | Siderophore receptors | Provides iron for microbial growth and survival | ||||||
| Efflux pumps | Remove antibiotics | |||||||
| Secretion systems | ||||||||
| Elastase, lipase, phospholipases, chitin-binding protein, exotoxin A, and others | Variety of proteolytic, lipolytic, and toxic factors; degrade host immune effectors | ||||||
| ExoS, ExoT, ExoU, ExoY | Intoxicates cells (ExoS, ExoT); cytotoxic (ExoU); disrupts actin cytoskeleton | ||||||
| Cytoplasmic and membrane-associated proteins, ATPases, lipoproteins, Hcp1 protein | Poorly characterized but found in animal studies to be needed for optimal virulence, particularly in chronic infection | ||||||
| Iron acquisition | Pyoverdin, pyochelin, HasAP | Scavenge iron from the host for bacterial use | ||||||
| Secreted toxins | Hemolysins, rhamnolipid phospholipases | Kill leukocytes, hemolysis of red cells, degrade host cell surface glycolipids | ||||||
| Secreted oxidative factors | Pyocyanin, ferric pyochelin, HCN | Produce reactive oxygen species: H | 2 | O | 2 | , O | 2− | Inflammatory, disrupts epithelial cell function |
| Quorum sensing | LasR/LasI, RhlR/RhlI, PQS | Biofilm formation, regulation of virulence factor secretion |
4. Antibodies as Therapeutics
Therapeutic antibodies work by activating and modulating our own host effector mechanisms including direct neutralization of toxins and pathogens, activation of the complement pathway, activation of neutrophil and macrophage opsonophagocytosis, activation of natural killer cells, enhancement of antigen presentation from dendritic cells to T cells and follicular dendritic cells to B cells, and degranulation of mast cells, eosinophils, and basophils. Additionally, Fc receptors that correspond to different classes of antibodies can activate the complement pathway, induce innate immune responses, and enhance natural adaptive immunity, providing a multi-faceted strategy to address disease [186][21]. Therapeutic monoclonal antibodies (mAbs) have emerged as a tour de force in the drug market and are projected to capture a rising percentage of the worldwide drug market—a trend that reflects increased prescription use of existing therapeutic mAbs and a large number (~50) of new therapeutic mAb drugs approved for use [187][22]. Currently, mAbs have been developed to treat a broad array of clinical conditions including cancer, autoimmune diseases, transplants, infectious disease, and toxin neutralization [188][23]. In contrast to mAbs, polyclonal antibodies (pAbs) have multiple epitope binding sites, thereby allowing greater coverage of neutralizing antigens. Much like our native immune system, pAbs are produced as a collection of antibodies from multiple B cell lineages, thus producing many different sites and affinities for the same antigen. Having multiple epitope binding sites allows for greater neutralization options and less opportunities for pathogens to develop escape mutants [189][24].Combinatorial Therapy
Recently, it has been shown that antibodies and antibiotics can act concomitantly or even synergistically against pathogens. Several studies have shown that exposure to antibiotic therapy alters the expression of secreted factors, cell surface proteins, and other cellular products that may be viable immunotargets [190][25]. These altered levels of expression can change and potentially enhance the innate and humoral immune responses to pathogens—namely through increased antigen exposure, increased protein secretion, and potentially decreased virulence of pathogens [190][25]. Inspired by these same principles, combination or conjugate antibiotic/antibody therapeutic strategies, particularly those based on polyclonal antibodies, could be designed to capitalize on these same altered expression levels to enhance therapeutic efficacy and combat resistance.References
- Kanj, S.; Sexton, M.D.; Daniel, M.D. Epidemiology, Microbiology, and Pathogenesis of Pseudomonas aeruginosa Infection. Available online: https://www.uptodate.com/contents/epidemiology-microbiology-and-pathogenesis-of-pseudomonas-aeruginosa-infection?search=pseudomonas&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2 (accessed on 21 September 2020).
- Owlia, P.; Nosrati, R.; Alaghehbandan, R.; Lari, A.R. Antimicrobial susceptibility differences among mucoid and non-mucoid Pseudomonas aeruginosa isolates. GMS Hyg. Infect. Control. 2014, 9, 13.
- Todar, K. Pseudomonas. Online Textbook of Bacteriology. Available online: http://textbookofbacteriology.net/pseudomonas_4.html (accessed on 21 September 2020).
- Souha Kanj, M.D. Principles of Antimicrobial Therapy of Pseudomonas aeruginosa Infections. Available online: https://www-uptodate-com.proxy.rvu.edu/contents/principles-of-antimicrobial-therapy-of-pseudomonas-aeruginosa-infections#H6675731 (accessed on 21 September 2020).
- Nordmann, P.; Poirel, L. Epidemiology and Diagnostics of Carbapenem Resistance in Gram-negative Bacteria. Clin Infect Dis. 2019, 69 (Suppl. S7), S521–S528.
- Bassetti, M.; Peghin, M.; Vena, A.; Giacobbe, D.R. Treatment of Infections Due to MDR Gram-Negative Bacteria. Front. Med. 2019, 6, 74.
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect Dis. 2020, 72, e169–e183.
- Grekov, I.; Thöming, J.G.; Kordes, A.; Häussler, S. Evolution of Pseudomonas aeruginosa toward higher fitness under standard laboratory conditions. ISME J. 2021, 15, 1165–1177.
- Fothergill, J.L.; Panagea, S.; Hart, C.A.; Walshaw, M.J.; Pitt, T.L.; Winstanley, C. Widespread pyocyanin over-production among isolates of a cystic fibrosis epidemic strain. BMC Microbiol. 2007, 7, 45.
- Mathee, K.; Ciofu, O.; Sternberg, C.; Lindum, P.W.; Campbell, J.I.A.; Jensen, P.; Johnsen, A.H.; Givskov, M.; Ohman, D.E.; Søren, M.; et al. Mucoid conversion of Pseudomonas aeruginos by hydrogen peroxide: A mechanism for virulence activation in the cystic fibrosis lung. Microbiology 1999, 145, 1349–1357.
- Von Götz, F.; Häussler, S.; Jordan, D.; Saravanamuthu, S.S.; Wehmhöner, D.; Strüssmann, A.; Lauber, J.; Attree, I.; Buer, J.; Tümmler, B.; et al. Expression Analysis of a Highly Adherent and Cytotoxic Small Colony Variant of Pseudomonas aeruginosa Isolated from a Lung of a Patient with Cystic Fibrosis. J. Bacteriol. 2004, 186, 3837.
- Häußler, S.; Tümmler, B.; Weißbrodt, H.; Rohde, M.; Steinmetz, I. Small-Colony Variants of Pseudomonas aeruginosa in Cystic Fibrosis. Clin. Infect. Dis. 1999, 29, 621–625.
- D’Argenio, D.A.; Calfee, M.W.; Rainey, P.B.; Pesci, E.C. Autolysis and Autoaggregation in Pseudomonas aeruginosa Colony Morphology Mutants. J. Bacteriol. 2002, 184, 6481.
- Smith, E.E.; Buckley, D.G.; Wu, Z.; Saenphimmachak, C.; Hoffman, L.R.; D’Argenio, D.A.; Miller, S.I.; Ramsey, B.W.; Speert, D.P.; Moskowitz, S.M.; et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 2006, 103, 8487–8492.
- Robak, O.H.; Heimesaat, M.M.; Kruglov, A.A.; Prepens, S.; Ninnemann, J.; Gutbier, B.; Reppe, K.; Hochrein, H.; Suter, M.; Kirschning, C.J.; et al. Antibiotic treatment-induced secondary IgA deficiency enhances susceptibility to Pseudomonas aeruginosa pneumonia. J. Clin. Investig. 2018, 128, 3535–3545.
- Schroeder, M.; Brooks, B.D.; Brooks, A.E. The Complex Relationship between Virulence and Antibiotic Resistance. Genes 2017, 8, 39.
- Mauch, R.M.; Jensen, P.Ø.; Moser, C.; Levy, C.E.; Høiby, N. Mechanisms of humoral immune response against Pseudomonas aeruginosa biofilm infection in cystic fibrosis. J. Cyst. Fibros. 2018, 17, 143–152.
- Meluleni, G.J.; Grout, M.; Evans, D.J.; Pier, G.B. Mucoid Pseudomonas aeruginosa growing in a biofilm in vitro are killed by opsonic antibodies to the mucoid exopolysaccharide capsule but not by antibodies produced during chronic lung infection in cystic fibrosis patients. J. Immunol. 1995, 155, 2029.
- Song, Z.; Wu, H.; Ciofu, O.; Kong, K.-F.; Høiby, N.; Rygaard, J.; Kharazmi, A.; Mathee, K. Pseudomonas aeruginosa alginate is refractory to Th1 immune response and impedes host immune clearance in a mouse model of acute lung infection. J. Med. Microbiol. 2003, 52, 731–740.
- Worlitzsch, D.; Tarran, R.; Ulrich, M.; Schwab, U.; Cekici, A.; Meyer, K.C.; Birrer, P.; Bellon, G.; Berger, J.; Weiss, T.; et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 2002, 109, 317–325.
- Hoffman, W.; Lakkis, F.G.; Chalasani, G. B Cells, Antibodies, and More. Clin. J. Am. Soc. Nephrol. CJASN 2016, 11, 137–154.
- Ascoli, C.A.; Aggeler, B. Overlooked benefits of using polyclonal antibodies. BioTechniques 2018, 65, 127–136.
- Arnold, J.N.; Wormald, M.R.; Sim, R.B.; Rudd, P.M.; Dwek, R.A. The Impact of Glycosylation on the Biological Function and Structure of Human Immunoglobulins. Annu. Rev. Immunol. 2007, 25, 21–50.
- Justiz Vaillant, A.A.; Ramphul, K. Immunoglobulin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: http://www.ncbi.nlm.nih.gov/books/NBK513460/ (accessed on 10 August 2020).
- Domenech, M.; Sempere, J.; de Miguel, S.; Yuste, J. Combination of Antibodies and Antibiotics as a Promising Strategy Against Multidrug-Resistant Pathogens of the Respiratory Tract. Front. Immunol. 2018, 9, 2700.
