Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Nora Tang and Version 1 by Rita Flores.
The main OCT biomarkers related to progression to advanced AMD include drusen volume, hyperreflective foci (HRF), reticular pseudodrusen or subretinal drusenoid deposits (SDD), incomplete retinal pigment epithelial and outer retinal atrophy (iRORA), hyper-transmission defects, and OCT-reflective drusen substructures (ODS).
- age related macular degeneration (AMD)
- drusen
- color fundus photography
- optical coherence tomography
1. Drusen Volume
In line with previous studies based on CFP, several investigators using spectral-domain OCT in patients with early and intermediate AMD reported that drusen area, height, and length predicted progression to advanced stages [15]. Eyes with higher baseline drusen volume have an increased risk of progression to either nAMD or GA [18], increase in drusen height is an important risk factor for progression to late atrophic AMD, and drusen length seems to have a positive correlation with the risk of conversion to nAMD [29].
More recent Spectral Domain-OCT (SD-OCT) volume studies provided more comprehensive measures [30,31,32,33,34,35]. Quantitative automatic determinations are available in some devices whose role can be useful in clinical practice. It is possible to automatically distinguish normal eyes from eyes with early and intermediate AMD by identifying total retinal volumes and RPE-drusen complex volumes.
Several studies have used automatic RPE-drusen complex (RPEDC) calculations with SD-OCT devices. Advanced RPE analysis in Cirrus HD-OCT platform provides the clinician with a rapid and reproducible quantitative approach for following disease progression in AMD patients (Advanced RPE analysis with RPEDC abnormal thickening and thinning) [36,37]. The RPEDC abnormal thickening and RPEDC abnormal thinning (RAT) volumes were generated by semiautomated segmentation within a 5 mm diameter macular field: macular OCT drusen volumes and RAT volumes increased significantly in AMD eyes over 2 years [36]. These quantitative SD-OCT biomarkers predict 2-year AMD progression and may serve as useful biomarkers for disease progression. Abnormal thinning of the RPE layer is believed to be an early precursor of atrophic lesions, as well as a marker of progression at the margins of expanding GA [36]. The RAT volume measurement improves specificity in distinguishing eyes with early precursors of GA from healthy control eyes, because control eyes did not demonstrate significant change in this parameter over 2 years [36].
Swept Source (SS) technology, with its longer wave-length and faster acquisition, provides better penetration below the RPE and therefore better visualization of the external layers of the retina and choroid. Improved visualization of external layers with SS-OCT devices, provides better automated segmentation performances, and more concordance in manual and automated measurements of drusen volume. Even more, some authors found no significant differences in drusen volume prior to and after manual correction in SS-OCT compared with a significant difference in SD-OCT devices [38].
2. Hyper-Reflective Foci
HRF are well defined, highly backscattering lesions within the neurosensory retina; they can be located adjacent to the drusen edge or apex (Figure 1A) or in the inner neurosensory retina. They are described as representing extracellular pigment granules and outer segment debris (outer HRF) or displacement and clumping of degenerated RPE cells or microglia (inner HRF). Curcio et al. hypothesized that anteriorly migrating RPE constitute one population of HRF and posteriorly migrating microglia the other [39].
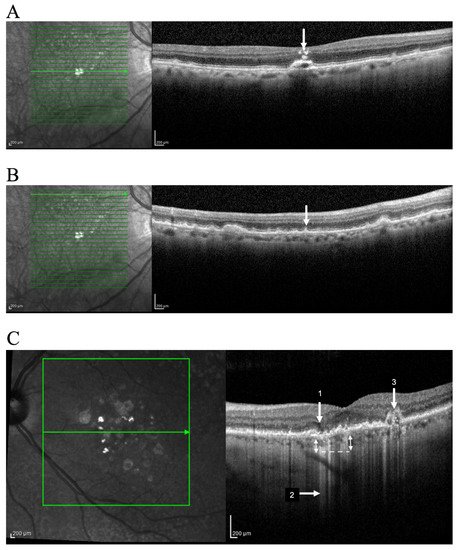
Figure 1. Identification of OCT progression biomarkers of AMD in cross sectional SD-OCT scan. Example of (A) Hyperreflective foci (arrow); (B) Subretinal drusenoid deposits (arrow); (C) iRORA (1); Hyper-transmission defects (2); OCT-reflective drusen substructures (ODS) (3). Representative imagens acquired using OCT SPECTRALIS (Heidelberg Engineering, Heidelberg, Germany).
RPE tends strongly to migrate anteriorly, suggesting either attractive signals from the retina or repellent signals from the drusen or both. One explanation is that cells at the druse apex are at maximum distance from the choriocapillaris and thus migrate into retina to seek oxygen from retinal vessels [39]. Another hypothesis considers that the high mechanical tension on the RPE layer at the drusen apex might serve to eject RPE cells [39].
HRF can appear as single or in clusters. They are relatively stable structures and considered a strong predictor of AMD progression [27]. The number and distribution of HRF across retinal layers are factors with predictive value in development of GA and nAMD, the latter being more associated with inner location and cluster distribution [27]. An extension of the AREDS2 study using SD-OCT demonstrated that patients with HRF on OCT at baseline had 5 times increased risk of progression to GA at 2 years when compared with controls [27].
Correlation with neovascular evolution is not so well demonstrated. Nevertheless, in nAMD, the regression of HRF after anti-VEGF treatment is considered a good functional prognostic sign. It was demonstrated that one month after the first injection, there was a statistically significant regression of HRF (p < 0.04) and an even more significant regression at the 3-month follow-up examination (p < 0.01). In the same study, there was a statistically significant reduction of HRF in the good and moderate visual acuity subgroups (respectively, p < 0.01 and p < 0.02). The persistence of HRD correlated significantly with the poor visual acuity group (p < 0.02) [40].
3. Reticular Pseudodrusen
These subretinal collections of granular, interlacing, hyper-reflective material located above RPE are commonly found in the superior macula or close to superotemporal arcades (Figure 1B). Observable by OCT, reticular pseudodrusen or SDD undergo a characteristic lifecycle of growth, invasion into the adjacent ellipsoid zone, and finally regression. Advanced stages in the SDD lifecycle are related to RPE degeneration and photoreceptor loss [41].
Infrared reflectance (IR) and SD-OCT appear to be particularly relevant methods to diagnose SDD. Using multimodal imaging, the prevalence of SDD appears higher than previously reported in studies based on retinal photography only. Regarding imaging techniques, the prevalence of SDD is lowest when diagnosed by CFP (6.7%) and fundus autofluorescence FAF (9.5%) and reaches the higher percentages when diagnosed with IR (18.1%) or SD-OCT (17.4%) [8].
Reticular pseudodrusen are found to be present in approximately 9% to 58% of patients with intermediate AMD, depending on the population studied [42,43]. In other studies, reticular pseudodrusen were present in 4.6% of eyes without AMD, 13.0% with early AMD, 62.6% with intermediate AMD, 34.6% with atrophic AMD, and 8.1% with neovascular AMD [8,42]. Researchers reported that the presence of reticular pseudodrusen is associated with an additional 2 to 6-fold increased risk of progression to nAMD or central GA, with the risk of progression higher for reticular pseudodrusen located outside the macula [42].
4. Incomplete Retinal Pigment Epithelial and Outer Retinal Atrophy
The term iRORA refers to the localized loss of tissue in the outer layers of retina without definite RPE loss. iRORA represent the new nomenclature obtained by the Consensus International Group (CAM—Classification of Atrophy Meeting) and has, more or less, replaced the so called “Nascent GA” [9,10,11,12,13]. Nascent GA and iRORA have not exactly the same meaning, because nascent GA should be only used in the absence of current or prior macular neovascularization.
iRORA represents a subsidence of the outer plexiform layer and the inner nuclear layer with a hypo-reflective wedge (Figure 1C) [44]. This finding is found in 22% of eyes with intermediate AMD and in 7% of all AMD patients [45]. iRORA is defined on OCT as: (1) a region of signal hypertransmission into the choroid; (2) a corresponding zone of attenuation or disruption of the RPE; and (3) evidence of overlying photoreceptor degeneration. The term iRORA should not be used when there is an RPE tear [11]. iRORA is strongly associated with impending GA as an average of 11 months elapsed between early signs of nascent GA and the development of GA [45].
Recognizing that photoreceptor atrophy can occur prior to RPE atrophy, and that atrophy can undergo different stages from outer retinal layer to RPE involvement, two previous stages were described in the atrophic progression: incomplete outer retinal atrophy (iORA) and complete outer retinal atrophy (cORA). This new classification opens novel reflection data in the atrophic pathologic development with progressive and complementary involvement of RPE and outer retinal layers cells.
In this new nomenclature, the term complete RPE and outer retinal atrophy (cRORA) was proposed as an end point for atrophy that occurred in the presence of drusen and was defined by the following criteria: (1) a region of hypertransmission of at least 250 mm in diameter; (2) a zone of attenuation or disruption of the RPE of at least 250 mm in diameter; and (3) evidence of overlying photoreceptor degeneration, all occurring in the absence of signs of an RPE tear [10].The CAM group believed that the term GA, well known in the AMD nomenclature and with several current CFP definitions, should remain and continue to be used as a term applied to the subset of cRORA occurring in the absence of current or past macular neovascularization [10].
Multimodal retinal imaging allows earlier and precise diagnose of atrophic AMD progression compared with isolated CFP. OCT identification of these different precursor lesions is an important issue in understanding the evolution to atrophic late stages [46].
5. Hyper-Transmission Defects
A commonly described OCT feature associated with atrophy is the presence of increased transmission of signal below the level of the RPE and into the choroid, resulting from loss of scatter or attenuation from overlying RPE and neurosensory retina.
A variety of terms were proposed including sub-RPE illumination, choroidal hyperreflectivity, narrow columns of hyper-reflectivity, and hyper-transmission defects. CAM participants agreed that the term hyper-transmission defects was the preferred term because it reports to the cause for the observed phenomenon and includes all cases even those with tall PEDs, where the hyper-transmission may not always penetrate to the underlying choroid [9,10,11,12,13].
Another common feature was a persistent hyperreflective line within the atrophy area, but significantly thinner than the adjacent RPE Bruch’s membrane band. This line is supposed to represent the so called “persistent basal laminar deposit” corresponding to a few dissociated RPE cells, as well as RPE granules, processes of Müller cells, and avascular fibrosis [47].
Finally, we can observe narrow columns of hyper-reflectivity under the RPE that suggest deficiencies in the RPE layer (Figure 1C) [48]. Prevalence is still unreported but some authors described sub-RPE hyper-reflective columns in 27% of eyes that progressed to nAMD or GA by at least three months [48]. These hyper-reflective columns are essentially hypertransmission in narrow strips, and their potential different meaning is not well defined.
6. OCT-Reflective Drusen Substructures
Variation in the OCT structure and properties of drusen has also been proposed to represent an increased risk of AMD progression. Drusen usually appear as a smooth, dome-shaped RPE elevation with homogeneous medium internal reflectivity and without HRF. These characteristics occur in about 47% of all drusen and only in 50% of the so-called soft drusen in CFP [17]. However, varying morphology, reflectivity, and internal homogeneity may be observed in other cases and these findings are important in monitoring intermediate AMD patients (Figure 1C).
A large multicenter study investigated such variations in patients with intermediate AMD enrolled in the AREDS2 SD-OCT study [49]. The authors found drusen substructures in 24% of intermediate AMD patients and described four phenotypic subtypes of variations or ODS: (a) low-reflective core (L type); (b) split-reflective core (S type); (c) high-reflective core (H type); and (d) conical debris (C type).
Presence of ODS at baseline in eyes with intermediate AMD was associated with progression to GA, but not to nAMD [49]. Low reflective cores may consist primarily of lipids, whereas high reflective cores may consist primarily of proteins and hydroxyapatite. Split drusen may simply be a heterogeneously composed drusen, with disease-associated molecules that have not seeded onto spherules, hence the split designation [49]. Conical debris may be calcified deposits caused by a shattering of high reflective cores. Conical debris (C type) is the subtype more closely related to atrophic changes, followed by H type, S type, and finally by L type [49].
In more recent studies, authors analyzed the heterogeneous internal reflectivity material within drusen (HIRD) and concluded it is formed by multilobular nodules composed of hydroxyapatite and that they are quite different from the spherules or refractile drusen, another refractile feature found in the retinas of patients with AMD [50]. Unlike spherules, which are small, refractile on CFP and reflective on OCT, nodules are large, refractile on CFP and nonreflective on OCT. Nodules are not simply aggregations of spherules and the heterogeneous HIRD should be called “calcified nodules” instead of the alternative terms “hyperreflective pyramidal structures” or conical debris [50]. These findings suggest that hydroxyapatite nodules may be indicators of progression to advanced AMD and that multimodal retinal imaging can help us to find outcome measures in AMD progression [50].
7. Other OCT Morphologic Findings
Ellipsoid zone disruption and focal RPE disruptions have also been associated with progression to advanced AMD and neovascularization [51]. Other features, independently associated with progression to advanced AMD, include presence of drusenoid PED, presence of RPE thickening, focal irregularity of the retina (thickening or thinning), irregularity or disruption of the external limiting membrane, and choroidal vessel abnormalities noted on OCT [10]. Table 1 presents a summary of the most common OCT progression biomarkers in AMD.
Table 1. Progression biomarkers in AMD.
| Biomarker | Imaging Findings | Mechanism(s) | Prevalence in AMD% | Expected Progression (OR 1) |
|---|---|---|---|---|
| Drusen volume | Baseline drusen volume | Displacement or deterioration of photoreceptor layer | ND 2 | 1.31 risk of progression to nAMD (for each 0.1 mm3 of drusen volume increase) [36] |
| RPE-Drusen complex (DC) Advanced analysis | RAT 3 | RPE suffering and drusen regression | ND 2 | 1.32 risk of developing central GA (for each 0.001 mm3 increase in RAT volume) [36] |
| HRF | Punctate hyperreflective lesions | Anterior migration of fully pigmentated RPE cells, inflammatory or microglia cell and calcification | 50% in AMD |
5 risk of 2-year progression to GA [27] |
| SDD | Small yellow deposits: reticular, ribbon-like or interdigitated | Dysfunction of cholesterol homeostasis, retinoid processing or choroidal hypoxia [55] | 32% to 79% in AMD patients |
2.24–3.4 risk of progression to advanced disease [1,42] |
| iRORA | Subsidence of the OPL 4 and INL 5 with a hypo-reflective wedge | New onset of atrophy (nascent atrophy) | 7% in intermediate AMD [56] | 5.2 risk of progression to central GA [45] |
| Hypertransmission | Columns or strips of hyperreflectivity | Deficiencies within RPE layer | 27% in AMD patients [48] | ND |
| ODS | Internal heterogeneity | Metabolic instability | 24% in soft drusen | 5.6 risk of progression to new atrophy onset [57] |
| Non exudative Retinal neovascularization | Neovascular lesion with no fluid | Protective mechanism against ischemia | 6.25 to 27% in the fellow eye of exudative AMD [54] | 1.21 risk of progression to exudative AMD at 1 year [52] |
1 Odds ratio; 2 not determined; 3 RPE Abnormal thinning; 4 Outer Plexiform Layer; 5 Inner nuclear Layer.
