Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Catherine Yang and Version 2 by Matilde Alique.
Kidney failure is also a major cause of cardiovascular morbidity and mortality. Indeed, epidemiological studies have demonstrated that chronic kidney disease (CKD) is a significant risk for cardiovascular events independently of classical risk.
- aging
- oxidative stress
- extracellular vesicles
- cardiorenal syndrome
- inflammation
- senescence
1. Age-Related Changes in Renal and Cardiovascular System
Even in the absence of other risk factors, the aging population presented structural and functional alterations in the kidneys, vessels, and heart. (Figure 1).
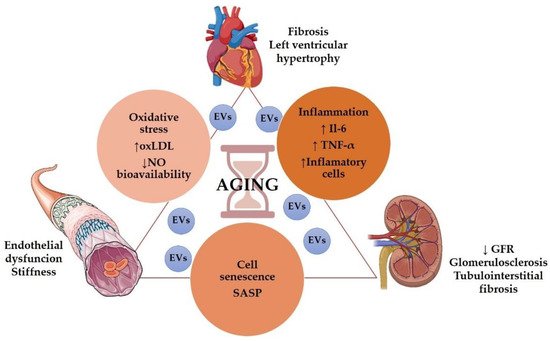
Figure 1. Role of aging in the cardiorenal syndrome. Some graphical elements from this figure were obtained from BioRender (http://biorender.com, accessed on 1 December 2021) and the Servier Medical ART (SMART) Powerpoint image bank (http://smart.servier.com, accessed on 1 December 2021).
Age is associated with a decrease in renal function [41][1], including glomerular filtration rate (GFR) decline and impaired urine concentrating capacity [42][2]. Even without any injury, GFR declined approximately 8 mL/min/1.73 m2 per decade after 40 years of age [43][3], but it has been suggested that GFR decline may start even earlier in the patient’s 20s [44][4]. Structurally, the aging kidney presented glomerulosclerosis, tubulointerstitial fibrosis, and tubular atrophy [45][5]. Besides, heart failure is classically related to the elderly [46][6]. An aging heart's most common pathophysiological characteristics are increased left ventricular (LV) hypertrophy and fibrosis [47][7]. Elderly patients presented diastolic dysfunction, increased atrial fibrillation, and a reduction in cardiac reserve [48][8]. Regarding vascular aging, it is characterized by endothelial dysfunction, mainly due to decreased nitric oxide (NO) availability [49][9], large arteries walls thickening, and progressive stiffness of central arteries, particularly the aorta [48[8][10],50], resulting in atherosclerosis Figure 1.
Several mechanisms may participate in aging-induced cardiovascular and renal structural and functional impairments, including cell senescence, inflammation, oxidative stress, and genetic and epigenetic modifications.
2. Cellular Senescence in Cardiovascular and Renal Aging
Cellular senescence is a process characterized by a stable cell–cycle arrest [51][11] that causes inflammation and the capacity to modify the microenvironment through the SASP. The accumulation of senescent cells in the kidney, heart and vascular vessels has been associated with structural and functional changes related to aging [52][12]. Furthermore, acquiring a senescent phenotype by aging or age-related chronic disease, including CKD and CKD-associated CVD, seems to be an irreversible pathophysiological process [22,53][13][14].
In large vessels, aging-associated endothelial cell senescence is a key cause of vascular structural changes and vascular dysfunction observed in atherosclerosis [45][5]. Additionally, human atherosclerotic plaque vulnerability is promoted by senescence vascular smooth muscle cells (VSMCs) [54][15]. Further osteoblastic-like phenotypes acquired by senescent VSMC seem to be responsible for vascular calcification [55][16]. In the kidney, the source of senescent cells depends on the pathology, and proximal tubular cells are the main source of senescent cells in the aged kidneys.
3. Inflammation in Cardiovascular and Renal Aging
Inflammaging describes the pro-inflammatory state observed in the older organism, even in the absence of other risk factors or diseases [13][17]. Several epidemiological studies have pointed to inflammaging as a risk factor of most age-related diseases, including CVD and CKD [56,57][18][19]. Chronic inflammation is a key factor in several CVD pathologies [58][20] and a pivotal contributor to CKD development and its progression to end-stage renal disease (ESRD) [59][21]. Moreover, proinflammatory cytokines and chemokines released by kidneys can reach the circulation, resulting in dysfunction of distant organs, including the cardiovascular system [60][22], a fact that may explain, at least in part, the accelerated cardiovascular aging observed in CKD patients [37][23].
Among the inflammatory mediators elevated in blood during aging, IL-6 and TNF-α are particularly noteworthy [61][24]. An elevation of both IL-6 and TNF-α and other molecules such as C-reactive proteins have been associated with high mortality in the elderly [62][25]. Even in centenarians, elevated levels of TNF-α correlated with morbidity, including CVD and mortality [63][26]. On the other hand, both molecules are also key factors in the onset and development of renal and CVDs [64,65,66,67][27][28][29][30]. Indeed, both molecules are considered uremic toxins and are therefore molecular markers and/or therapeutic targets for the cardiorenal syndrome.
Elevated IL-6 and TNF-α levels have been observed in CKD patients, and these levels are inversely correlated with GFR [68][31]. Moreover, high IL-6 levels and TNF-α have been associated with the development of atherosclerosis and vascular calcification in CKD patients [69,70,71][32][33][34]. Likewise, IL-6 has been proposed as a risk factor for left ventricular hypertrophy in peritoneal dialysis patients [72][35].
The human GG polymorphism at the −174 position in the promoter region of the IL-6 gene, which is associated with increased levels of IL-6, has been related to an increased risk of developing age-associated CVD [73,74][36][37] and with increased mortality in peritoneal dialysis patients [75][38]. On the other hand, this polymorphism is less frequent in centenarians than in young adults [76][39], whereas other IL-6 SNPs have been associated with longevity [77,78][40][41]. Moreover, in aged patients, including centenarians, high levels of TNF-α in the blood were associated with a high prevalence of atherosclerosis [63,79][26][42].
4. Oxidative Stress in Cardiovascular and Renal Aging
As previously indicated, the oxidative stress theory of aging states that age-associated loss of functionality would be due to the accumulation of oxidative damage to lipids, DNA, and proteins by ROS and RNS [5][43]. However, recent studies have demonstrated a more complex relation between oxidant and antioxidant mechanisms in aging and age-related diseases [80][44].
Oxidative stress is a key component of several age-related pathologies, including CVDs and acute CKD. The role of different pro-oxidant molecules and the therapeutic effects of several antioxidants have been widely studied in experimental models and clinical trials [81,82,83,84,85,86,87,88][45][46][47][48][49][50][51][52].
CKD and ESRD patients show increased levels of different oxidative stress markers, including advanced oxidation protein products, malondialdehyde, and oxidized low-density lipoproteins (ox-LDL), which have been associated with a decline in renal function. Furthermore, an increase in ox-LDL, together with high IL-6 levels, has been associated with an increased risk of CVD events and CVD-related mortality in CKD patients in hemodialysis (HD) [89][53] and accelerated atherosclerosis development observed in CKD [82][46]. Besides its role in foam cells formation within the arterial wall, ox-LDLs also participate in other proatherogenic events, including endothelial dysfunction and smooth muscle proliferation, suggesting an essential role of ox-LDLs in atherosclerotic plaque development and destabilization [90,91][54][55].
Furthermore, increased levels of ox-LDL in older adults have also been associated with arterial stiffening [92][56]. However, another study in aged patients reported no correlation between ox-LDL levels and cardiovascular morbidity nor mortality, suggesting that in elderly patients, the ox-LDL may not be a good marker [93][57]. What seems clear is that ox-LDL levels are related to endothelial dysfunction observed in adults and elderly individuals [49][9].
Oxidative stress induces endothelial dysfunction by decreasing NO bioavailability [94][58], mainly by the formation of peroxynitrite (ONOO−), through its combination with superoxide anion (O2•−), which is elevated in atherosclerotic lesions [95][59]. Moreover, ONOO− leads to endothelial nitric oxide synthase (eNOS) uncoupling activity, thus perpetuating the detrimental response. In addition to its role in endothelial function, NO has other effects, including antithrombotic, anti-inflammatory, and anti-atherogenic effects [49][9]. Therefore, in vascular endothelium, ox-LDL and NO exert antagonistic actions in all phases of atherogenesis. Indeed, some authors have proposed using ox-LDL to NO ratio (ox-LDL/NO) as a new biomarker for endothelial dysfunction in atherosclerosis [49][9]. Curiously, whereas NO produced by eNOS seems to have atheroprotective effects, excessive NO produced by inducible nitric oxide synthase (iNOS), under proinflammatory conditions, had a detrimental impact on the endothelium [95][59]. Conversely, elderly humans presented elevated NO production within the vasculature but a reduced NO bioavailability. In the kidney, aging-associated NO reduction increases renal vascular vasoconstriction, Na+ retention, and renal fibrosis, thus contributing to enhanced hypertension and declined renal function [45][5].
Finally, given the close relationship between oxidative stress, inflammation, and aging, the free radical theory of aging has been updated, giving rise to the oxidation-inflammatory theory of aging or oxi-inflamm-aging [96][60]. This new theory postulates that aging is a loss of body homeostasis due to sustained oxidative stress that activates different systems, including the immune system, thus inducing an inflammatory response that increases oxidative stress and perpetuates positive feedback of oxidative stress and inflammation.
5. Extracellular Vesicles in Cardiovascular and Renal Aging
In human renal and cardiovascular pathologies, EVs' changes in composition and levels have been described [97][61]. In addition, different studies showed the effect of drug treatment on EVs’ profile in other diseases [98][62]. Altogether these results point to a potential role of EVs as biomarkers for diagnosis and as tools for therapy by drug administration of different cargo.
In CKD, circulating EVs are augmented and are key players in vascular calcification [99][63], endothelial dysfunction [100][64], and vascular mortality [101][65]. In hemodialyzed patients with CKD, plasma circulating EVs were increased compared with elderly subjects without CKD used as controls [102][66]. In this study, the level of EVs released by proinflammatory monocytes was high, and no differences in total monocyte-derived EVs were found as other authors had previously described [103,104,105][67][68][69]. The uremic toxin proinflammatory environment in these CKD patients induces proinflammatory monocytes activation, alters miR-126-3p, miR-233-3p, miR-192-5p expression, and increases the release of proinflammatory EVs that enhance vascular inflammation. As miR-126-3p participates in endothelial proliferation and endothelization in large vessels [106,107[70][71][72],108], the decreased miR-126-3p circulating levels reported in these hemodialyzed patients indicate its implication in the vascular dysfunction observed [102][66].
In addition, the decrease in miRNA-233-3p expression and circulating levels observed in CKD patients was reversed and even increased after kidney transplantation [109,110][73][74], indicating its participation in vascular complications development. The lower expression of miR-192-5p was also found in hemodialyzed patients [102][66], venous thromboembolism [111][75], and hypertension [112][76]. As the expression of several miRNAs can be positively or negatively correlated with different diseases and inflammatory states, authors consider those miRNA ratios to be a clinical feature of every disease and a diagnostic and therapeutic biomarker.
The mentioned studies suggest that serum levels and the profile of miRNAs and EVs depend on CKD’s uremic inflammatory state and promote cardiovascular damage [102][66].
The progressive decrease in renal function is a risk factor in most CVDs and worsens the clinical outcomes [113,114][77][78].
For example, non-valvular atrial fibrillation is linked to kidney disease because of increased thromboembolism mediated by higher EV levels from the prothrombotic endothelial-platelet origin but not by other markers of thrombotic state and cellular activation [115][79] even in anticoagulated patients.
In hypertensive patients, the presence of EVs indicating podocyte injury, a characteristic expression of miRNAs, and peritubular capillaries damage has been described [116][80]. Furthermore, EVs released by endothelial cells from perivascular capillaries had been detected in the urine of essential and renovascular hypertensive patients, the concentration of which directly correlates with clinical parameters and capillary rarefaction but inversely with renal perfusion [117][81]. Therefore, the levels of urinary EVs in hypertension could be an early marker of renal injury due to peritubular capillaries damage, and the said levels inversely correlate with renal function (estimated glomerular filtration, eGFR) after medical treatment in essential and renovascular hypertensive patients [117][81].
Intensive treatment of T2DM patients suffering an acute coronary attack showed decreased endothelial CD31+/CD41+ EVs levels [118][82]. Administration of pioglitazone to patients with metabolic syndrome reduced endothelial EV levels [119][83]. In patients with T2DM and hypertension, endothelial EV levels correlate directly with the mean systolic and pulse blood pressure but inversely with eGFR compared with normotensive diabetic patients [120][84]. CD31+/CD42− [121,122][85][86] and CD31+/CD42−/CD51+ [123][87] endothelial-derived EVs are increased in hypertensive patients with T2DM correlating these levels with mean arterial pressure and mean systolic blood pressure.
Endothelial-derived EVs can be considered an endothelial damage marker from the studies explained above. In addition, along with EVs secreted from other sources such as platelets and leukocytes, endothelial-derived EVs play an active role in the pathogenesis of hypertension. Furthermore, increased levels of EVs relate to a minor ability of vessels to regenerate, increasing cardiovascular risk and nephropathy [124][88]. All these studies point to the importance of assessing plasma EV levels to establish the risk of organ damage in diabetes.
In a different approach, EVs would have beneficial effects as carriers of signals to preserve, for example, endothelial function and vessel integrity in vascular diseases [125,126][89][90]. Indeed, EVs have therapeutic potential as vehicles for transferring and secreting different molecules (cytokines, chemokines, growth factors, nucleic acids, etc.) to other targets in disease [127][91]. Furthermore, EVs from mesenchymal stem cells can preserve myocardial function after ischemia/reperfusion in animal models and humans [128,129,130][92][93][94]. Moreover, EVs derived from bone marrow CD34+, or endothelial progenitor cells, increase cardiac viability by decreasing oxidative stress and activating PI3K/Akt pathway, and promoting angiogenesis [131,132,133][95][96][97]. In addition, EVs derived from cardiac progenitor cells protect the myocardium from ischemia/reperfusion injury [134][98].
EVs have advantages in regenerative medicine and therapy because they maintain their properties during long storage periods. Thus, the limitations of using viable cells that can undergo aberrant differentiation are avoided.
EVs could be helpful to vectors in gene therapy by transporting and delivering nucleic acids. For instance, PI3K/Akt pathway mRNAs carried by endothelial progenitor cells-derived EVs promote angiogenesis response in endothelial cells after EVs and endothelial cell fusion [135][99]. Circulating EVs also carry miRNAs known for their implication in the pathophysiology of cardiovascular and other diseases by modulating target cell gene expression [136][100], and specific miRNAs are expressed and packed in circulating EVs in these diseases [137][101]. This compartmentalization is stimulus-dependent. This is similar to hypoxia which determines the regenerative properties of mesenchymal stem cells-derived EVs and the expression of pro-angiogenic miRNAs in endothelial progenitor cells-derived EVs [128,138,139][92][102][103]. In addition, it has been demonstrated that miR-126 is carried by circulating EVs for regulating angiogenesis and vascular integrity [108,140,141][72][104][105]. miR-126 transported into recipient human coronary artery endothelial cells by endothelial EVs released by apoptotic endothelial cells promoted reendothelialization. Still, hyperglycemia lowered the amounts of miR-126 transported and reduced endothelial repair capacity in vivo [141][105]. Interestingly, patients with coronary artery disease have low levels or lack miR-126 compared with healthy subjects [138,139][102][103], indicating the importance of EVs cargo in developing and treating the disease.
References
- Davies, D.F.; Shock, N.W. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J. Clin. Investig. 1950, 29, 496–507.
- O’Sullivan, E.D.; Hughes, J.; Ferenbach, D.A. Renal Aging: Causes and Consequences. J. Am. Soc. Nephrol. 2017, 28, 407–420.
- Hoy, W.E.; Douglas-Denton, R.N.; Hughson, M.D.; Cass, A.; Johnson, K.; Bertram, J.F. A stereological study of glomerular number and volume: Preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int. 2003, 63, S31–S37.
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Levey, A.S. Prevalence of chronic kidney disease in the United States. JAMA 2007, 298, 2038–2047.
- Weinstein, J.R.; Anderson, S. The aging kidney: Physiological changes. Adv. Chronic Kidney Dis. 2010, 17, 302–307.
- McMurray, J.J.; Petrie, M.C.; Murdoch, D.R.; Davie, A.P. Clinical epidemiology of heart failure: Public and private health burden. Eur. Heart J. 1998, 19, 9–16.
- Chiao, Y.A.; Rabinovitch, P.S. The Aging Heart. Cold Spring Harb. Perspect. Med. 2015, 5, a025148.
- Lakatta, E.G. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part III: Cellular and molecular clues to heart and arterial aging. Circulation 2003, 107, 490–497.
- Gradinaru, D.; Borsa, C.; Ionescu, C.; Prada, G.I. Oxidized LDL and NO synthesis--Biomarkers of endothelial dysfunction and ageing. Mech. Ageing Dev. 2015, 151, 101–113.
- Paneni, F.; Diaz Cañestro, C.; Libby, P.; Lüscher, T.F.; Camici, G.G. The Aging Cardiovascular System: Understanding It at the Cellular and Clinical Levels. J. Am. Coll. Cardiol. 2017, 69, 1952–1967.
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827.
- Valentijn, F.A.; Falke, L.L.; Nguyen, T.Q.; Goldschmeding, R. Cellular senescence in the aging and diseased kidney. J. Cell Commun. Signal. 2018, 12, 69–82.
- Carracedo, J.; Ramírez-carracedo, R.; Alique, M.; Ramírez-Chamond, R. Endothelial Cell Senescence in the Pathogenesis of Endothelial Dysfunction. In Endothelial Dysfunction—Old Concepts and New Challenges; Lenasi, H., Ed.; IntechOpen: London, UK, 2018.
- Dai, L.; Qureshi, A.R.; Witasp, A.; Lindholm, B.; Stenvinkel, P. Early Vascular Ageing and Cellular Senescence in Chronic Kidney Disease. Comput. Struct. Biotechnol. J. 2019, 17, 721–729.
- Wang, J.; Uryga, A.K.; Reinhold, J.; Figg, N.; Baker, L.; Finigan, A.; Gray, K.; Kumar, S.; Clarke, M.; Bennett, M. Vascular Smooth Muscle Cell Senescence Promotes Atherosclerosis and Features of Plaque Vulnerability. Circulation 2015, 132, 1909–1919.
- Burton, D.G.; Matsubara, H.; Ikeda, K. Pathophysiology of vascular calcification: Pivotal role of cellular senescence in vascular smooth muscle cells. Exp. Gerontol. 2010, 45, 819–824.
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254.
- Fried, L.; Solomon, C.; Shlipak, M.; Seliger, S.; Stehman-Breen, C.; Bleyer, A.J.; Chaves, P.; Furberg, C.; Kuller, L.; Newman, A. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J. Am. Soc. Nephrol. 2004, 15, 3184–3191.
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522.
- Yang, C.; Deng, Z.; Li, J.; Ren, Z.; Liu, F. Meta-analysis of the relationship between interleukin-6 levels and the prognosis and severity of acute coronary syndrome. Clinics 2021, 76, e2690.
- Amdur, R.L.; Feldman, H.I.; Gupta, J.; Yang, W.; Kanetsky, P.; Shlipak, M.; Rahman, M.; Lash, J.P.; Townsend, R.R.; Ojo, A.; et al. Inflammation and Progression of CKD: The CRIC Study. Clin. J. Am. Soc. Nephrol. 2016, 11, 1546–1556.
- Rosner, M.H.; Ronco, C.; Okusa, M.D. The role of inflammation in the cardio-renal syndrome: A focus on cytokines and inflammatory mediators. Semin. Nephrol. 2012, 32, 70–78.
- Stenvinkel, P.; Larsson, T.E. Chronic kidney disease: A clinical model of premature aging. Am. J. Kidney Dis. 2013, 62, 339–351.
- Singh, T.; Newman, A.B. Inflammatory markers in population studies of aging. Ageing Res. Rev. 2011, 10, 319–329.
- Harris, T.B.; Ferrucci, L.; Tracy, R.P.; Corti, M.C.; Wacholder, S.; Ettinger, W.H., Jr.; Heimovitz, H.; Cohen, H.J.; Wallace, R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med. 1999, 106, 506–512.
- Bruunsgaard, H.; Andersen-Ranberg, K.; Hjelmborg, J.; Pedersen, B.K.; Jeune, B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am. J. Med. 2003, 115, 278–283.
- Chen, W.; Yuan, H.; Cao, W.; Wang, T.; Yu, H.; Fu, Y.; Jiang, B.; Zhou, H.; Guo, H.; Zhao, X. Blocking interleukin-6 trans-signaling protects against renal fibrosis by suppressing STAT3 activation. Theranostics 2019, 9, 3980–3991.
- Hashmat, S.; Rudemiller, N.; Lund, H.; Abais-Battad, J.M.; Van Why, S.; Mattson, D.L. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am. J. Physiol. Ren. Physiol. 2016, 311, F555–F561.
- Zhang, W.; Wang, W.; Yu, H.; Zhang, Y.; Dai, Y.; Ning, C.; Tao, L.; Sun, H.; Kellems, R.E.; Blackburn, M.R.; et al. Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension 2012, 59, 136–144.
- Lim, Y.J.; Sidor, N.A.; Tonial, N.C.; Che, A.; Urquhart, B.L. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets. Toxins 2021, 13, 142.
- Spoto, B.; Leonardis, D.; Parlongo, R.M.; Pizzini, P.; Pisano, A.; Cutrupi, S.; Testa, A.; Tripepi, G.; Zoccali, C.; Mallamaci, F. Plasma cytokines, glomerular filtration rate and adipose tissue cytokines gene expression in chronic kidney disease (CKD) patients. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 981–988.
- Hassan, M.O.; Duarte, R.; Dickens, C.; Dix-Peek, T.; Naidoo, S.; Vachiat, A.; Grinter, S.; Manga, P.; Naicker, S. Interleukin-6 gene polymorhisms and interleukin-6 levels are associated with atherosclerosis in CKD patients. Clin. Nephrol. 2020, 93, 82–86.
- Stenvinkel, P.; Heimbürger, O.; Paultre, F.; Diczfalusy, U.; Wang, T.; Berglund, L.; Jogestrand, T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999, 55, 1899–1911.
- Schlieper, G.; Schurgers, L.; Brandenburg, V.; Reutelingsperger, C.; Floege, J. Vascular calcification in chronic kidney disease: An update. Nephrol. Dial. Transplant. 2016, 31, 31–39.
- Liu, W.H.; Zhou, Q.L.; Ao, X.; Yu, H.L.; Peng, W.S.; He, N. Fibroblast growth factor-23 and interleukin-6 are risk factors for left ventricular hypertrophy in peritoneal dialysis patients. J. Cardiovasc. Med. 2012, 13, 565–569.
- Hou, H.; Wang, C.; Sun, F.; Zhao, L.; Dun, A.; Sun, Z. Association of interleukin-6 gene polymorphism with coronary artery disease: An updated systematic review and cumulative meta-analysis. Inflamm. Res. 2015, 64, 707–720.
- Humphries, S.E.; Luong, L.A.; Ogg, M.S.; Hawe, E.; Miller, G.J. The interleukin-6 -174 G/C promoter polymorphism is associated with risk of coronary heart disease and systolic blood pressure in healthy men. Eur. Heart J. 2001, 22, 2243–2252.
- Verduijn, M.; Maréchal, C.; Coester, A.M.; Sampimon, D.E.; Boeschoten, E.W.; Dekker, F.W.; Goffin, E.; Krediet, R.T.; Devuyst, O. The -174G/C variant of IL6 as risk factor for mortality and technique failure in a large cohort of peritoneal dialysis patients. Nephrol. Dial. Transplant. 2012, 27, 3516–3523.
- Rea, I.M.; Ross, O.A.; Armstrong, M.; McNerlan, S.; Alexander, D.H.; Curran, M.D.; Middleton, D. Interleukin-6-gene C/G 174 polymorphism in nonagenarian and octogenarian subjects in the BELFAST study. Reciprocal effects on IL-6, soluble IL-6 receptor and for IL-10 in serum and monocyte supernatants. Mech. Ageing Dev. 2003, 124, 555–561.
- Soerensen, M.; Dato, S.; Tan, Q.; Thinggaard, M.; Kleindorp, R.; Beekman, M.; Suchiman, H.E.; Jacobsen, R.; McGue, M.; Stevnsner, T.; et al. Evidence from case-control and longitudinal studies supports associations of genetic variation in APOE, CETP, and IL6 with human longevity. Age 2013, 35, 487–500.
- Zeng, Y.; Nie, C.; Min, J.; Liu, X.; Li, M.; Chen, H.; Xu, H.; Wang, M.; Ni, T.; Li, Y.; et al. Novel loci and pathways significantly associated with longevity. Sci. Rep. 2016, 6, 21243.
- Bruunsgaard, H.; Skinhøj, P.; Pedersen, A.N.; Schroll, M.; Pedersen, B.K. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin. Exp. Immunol. 2000, 121, 255–260.
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772.
- Santos, A.L.; Sinha, S.; Lindner, A.B. The Good, the Bad, and the Ugly of ROS: New Insights on Aging and Aging-Related Diseases from Eukaryotic and Prokaryotic Model Organisms. Oxid. Med. Cell. Longev. 2018, 2018, 1941285.
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752.
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubiña, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. 2008, 74, S4–S9.
- Sakashita, M.; Tanaka, T.; Inagi, R. Metabolic Changes and Oxidative Stress in Diabetic Kidney Disease. Antioxidants 2021, 10, 1143.
- Zhou, Y.; Murugan, D.D.; Khan, H.; Huang, Y.; Cheang, W.S. Roles and Therapeutic Implications of Endoplasmic Reticulum Stress and Oxidative Stress in Cardiovascular Diseases. Antioxidants 2021, 10, 1167.
- Martin-Ventura, J.L.; Rodrigues-Diez, R.; Martinez-Lopez, D.; Salaices, M.; Blanco-Colio, L.M.; Briones, A.M. Oxidative Stress in Human Atherothrombosis: Sources, Markers and Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 2315.
- Düsing, P.; Zietzer, A.; Goody, P.R.; Hosen, M.R.; Kurts, C.; Nickenig, G.; Jansen, F. Vascular pathologies in chronic kidney disease: Pathophysiological mechanisms and novel therapeutic approaches. J. Mol. Med. 2021, 99, 335–348.
- Ogura, Y.; Kitada, M.; Koya, D. Sirtuins and Renal Oxidative Stress. Antioxidants 2021, 10, 1198.
- Stenvinkel, P.; Chertow, G.M.; Devarajan, P.; Levin, A.; Andreoli, S.P.; Bangalore, S.; Warady, B.A. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int. Rep. 2021, 6, 1775–1787.
- Honda, H.; Ueda, M.; Kojima, S.; Mashiba, S.; Michihata, T.; Takahashi, K.; Shishido, K.; Akizawa, T. Oxidized high-density lipoprotein as a risk factor for cardiovascular events in prevalent hemodialysis patients. Atherosclerosis 2012, 220, 493–501.
- Yoshimoto, R.; Fujita, Y.; Kakino, A.; Iwamoto, S.; Takaya, T.; Sawamura, T. The discovery of LOX-1, its ligands and clinical significance. Cardiovasc. Drugs Ther. 2011, 25, 379–391.
- Sawamura, T.; Kume, N.; Aoyama, T.; Moriwaki, H.; Hoshikawa, H.; Aiba, Y.; Tanaka, T.; Miwa, S.; Katsura, Y.; Kita, T.; et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 1997, 386, 73–77.
- Brinkley, T.E.; Nicklas, B.J.; Kanaya, A.M.; Satterfield, S.; Lakatta, E.G.; Simonsick, E.M.; Sutton-Tyrrell, K.; Kritchevsky, S.B. Plasma oxidized low-density lipoprotein levels and arterial stiffness in older adults: The health, aging, and body composition study. Hypertension 2009, 53, 846–852.
- Zuliani, G.; Morieri, M.L.; Volpato, S.; Vigna, G.B.; Bosi, C.; Maggio, M.; Cherubini, A.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. Determinants and clinical significance of plasma oxidized LDLs in older individuals. A 9 years follow-up study. Atherosclerosis 2013, 226, 201–207.
- Münzel, T.; Heitzer, T.; Harrison, D.G. The physiology and pathophysiology of the nitric oxide/superoxide system. Herz 1997, 22, 158–172.
- Chatterjee, A.; Black, S.M.; Catravas, J.D. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul. Pharmacol. 2008, 49, 134–140.
- De la Fuente, M.; Miquel, J. An update of the oxidation-inflammation theory of aging: The involvement of the immune system in oxi-inflamm-aging. Curr. Pharm. Des. 2009, 15, 3003–3026.
- Amabile, N.; Cheng, S.; Renard, J.M.; Larson, M.G.; Ghorbani, A.; McCabe, E.; Griffin, G.; Guerin, C.; Ho, J.E.; Shaw, S.Y.; et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur. Heart J. 2014, 35, 2972–2979.
- Martinez, M.C.; Tual-Chalot, S.; Leonetti, D.; Andriantsitohaina, R. Microparticles: Targets and tools in cardiovascular disease. Trends Pharmacol. Sci. 2011, 32, 659–665.
- Dickhout, A.; Koenen, R.R. Extracellular Vesicles as Biomarkers in Cardiovascular Disease; Chances and Risks. Front. Cardiovasc. Med. 2018, 5, 113.
- Amabile, N.; Guérin, A.P.; Leroyer, A.; Mallat, Z.; Nguyen, C.; Boddaert, J.; London, G.M.; Tedgui, A.; Boulanger, C.M. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J. Am. Soc. Nephrol. 2005, 16, 3381–3388.
- Carmona, A.; Agüera, M.L.; Luna-Ruiz, C.; Buendía, P.; Calleros, L.; García-Jerez, A.; Rodríguez-Puyol, M.; Arias, M.; Arias-Guillen, M.; de Arriba, G.; et al. Markers of endothelial damage in patients with chronic kidney disease on hemodialysis. Am. J. Physiol. Ren. Physiol. 2017, 312, F673–F681.
- Carmona, A.; Guerrero, F.; Jimenez, M.J.; Ariza, F.; Agüera, M.L.; Obrero, T.; Noci, V.; Muñoz-Castañeda, J.R.; Rodríguez, M.; Soriano, S.; et al. Inflammation, Senescence and MicroRNAs in Chronic Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 739.
- Eikendal, A.L.; den Ruijter, H.M.; Uiterwaal, C.S.; Pasterkamp, G.; Hoefer, I.E.; de Kleijn, D.P.; Schoneveld, A.H.; Leiner, T.; Bots, M.L.; Visseren, F.L.; et al. Extracellular vesicle protein CD14 relates to common carotid intima-media thickness in eight-year-old children. Atherosclerosis 2014, 236, 270–276.
- Kanhai, D.A.; Visseren, F.L.; van der Graaf, Y.; Schoneveld, A.H.; Catanzariti, L.M.; Timmers, L.; Kappelle, L.J.; Uiterwaal, C.S.; Lim, S.K.; Sze, S.K.; et al. Microvesicle protein levels are associated with increased risk for future vascular events and mortality in patients with clinically manifest vascular disease. Int. J. Cardiol. 2013, 168, 2358–2363.
- Vrijenhoek, J.E.; Pasterkamp, G.; Moll, F.L.; de Borst, G.J.; Bots, M.L.; Catanzariti, L.; van de Weg, S.M.; de Kleijn, D.P.; Visseren, F.L.; Ruijter, H.M. Extracellular vesicle-derived CD14 is independently associated with the extent of cardiovascular disease burden in patients with manifest vascular disease. Eur. J. Prev. Cardiol. 2015, 22, 451–457.
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, Iii27-32.
- Economou, E.K.; Oikonomou, E.; Siasos, G.; Papageorgiou, N.; Tsalamandris, S.; Mourouzis, K.; Papaioanou, S.; Tousoulis, D. The role of microRNAs in coronary artery disease: From pathophysiology to diagnosis and treatment. Atherosclerosis 2015, 241, 624–633.
- Zernecke, A.; Bidzhekov, K.; Noels, H.; Shagdarsuren, E.; Gan, L.; Denecke, B.; Hristov, M.; Köppel, T.; Jahantigh, M.N.; Lutgens, E.; et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2009, 2, ra81.
- Fourdinier, O.; Schepers, E.; Metzinger-Le Meuth, V.; Glorieux, G.; Liabeuf, S.; Verbeke, F.; Vanholder, R.; Brigant, B.; Pletinck, A.; Diouf, M.; et al. Serum levels of miR-126 and miR-223 and outcomes in chronic kidney disease patients. Sci. Rep. 2019, 9, 4477.
- Ulbing, M.; Kirsch, A.H.; Leber, B.; Lemesch, S.; Münzker, J.; Schweighofer, N.; Hofer, D.; Trummer, O.; Rosenkranz, A.R.; Müller, H.; et al. MicroRNAs 223-3p and 93-5p in patients with chronic kidney disease before and after renal transplantation. Bone 2017, 95, 115–123.
- Starikova, I.; Jamaly, S.; Sorrentino, A.; Blondal, T.; Latysheva, N.; Sovershaev, M.; Hansen, J.B. Differential expression of plasma miRNAs in patients with unprovoked venous thromboembolism and healthy control individuals. Thromb. Res. 2015, 136, 566–572.
- Berillo, O.; Huo, K.G.; Fraulob-Aquino, J.C.; Richer, C.; Briet, M.; Boutouyrie, P.; Lipman, M.L.; Sinnett, D.; Paradis, P.; Schiffrin, E.L. Circulating let-7g-5p and miR-191-5p Are Independent Predictors of Chronic Kidney Disease in Hypertensive Patients. Am. J. Hypertens. 2020, 33, 505–513.
- Bonde, A.N.; Lip, G.Y.; Kamper, A.L.; Hansen, P.R.; Lamberts, M.; Hommel, K.; Hansen, M.L.; Gislason, G.H.; Torp-Pedersen, C.; Olesen, J.B. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: A nationwide observational cohort study. J. Am. Coll. Cardiol. 2014, 64, 2471–2482.
- Olesen, J.B.; Lip, G.Y.; Kamper, A.L.; Hommel, K.; Køber, L.; Lane, D.A.; Lindhardsen, J.; Gislason, G.H.; Torp-Pedersen, C. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N. Engl. J. Med. 2012, 367, 625–635.
- Lau, Y.C.; Xiong, Q.; Blann, A.D.; Lip, G.Y. Relationship between renal function and circulating microparticles, soluble P-selectin and E-selectin levels in atrial fibrillation. J. Thromb. Thrombolysis 2017, 43, 18–23.
- Zhou, H.; Kajiyama, H.; Tsuji, T.; Hu, X.; Leelahavanichkul, A.; Vento, S.; Frank, R.; Kopp, J.B.; Trachtman, H.; Star, R.A.; et al. Urinary exosomal Wilms’ tumor-1 as a potential biomarker for podocyte injury. Am. J. Physiol. Ren. Physiol. 2013, 305, F553–F559.
- Sun, I.O.; Santelli, A.; Abumoawad, A.; Eirin, A.; Ferguson, C.M.; Woollard, J.R.; Lerman, A.; Textor, S.C.; Puranik, A.S.; Lerman, L.O. Loss of Renal Peritubular Capillaries in Hypertensive Patients Is Detectable by Urinary Endothelial Microparticle Levels. Hypertension 2018, 72, 1180–1188.
- Lumsden, N.G.; Andrews, K.L.; Bobadilla, M.; Moore, X.L.; Sampson, A.K.; Shaw, J.A.; Mizrahi, J.; Kaye, D.M.; Dart, A.M.; Chin-Dusting, J.P. Endothelial dysfunction in patients with type 2 diabetes post acute coronary syndrome. Diabetes Vasc. Dis. Res. 2013, 10, 368–374.
- Esposito, K.; Ciotola, M.; Giugliano, D. Pioglitazone reduces endothelial microparticles in the metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1926.
- Dec-Gilowska, M.; Trojnar, M.; Makaruk, B.; Czop, M.; Przybylska-Kuc, S.; Mosiewicz-Madejska, B.; Dzida, G.; Mosiewicz, J. Circulating Endothelial Microparticles and Aortic Stiffness in Patients with Type 2 Diabetes Mellitus. Medicina 2019, 55, 596.
- Chen, Y.; Feng, B.; Li, X.; Ni, Y.; Luo, Y. Plasma endothelial microparticles and their correlation with the presence of hypertension and arterial stiffness in patients with type 2 diabetes. J. Clin. Hypertens. 2012, 14, 455–460.
- Feng, B.; Chen, Y.; Luo, Y.; Chen, M.; Li, X.; Ni, Y. Circulating level of microparticles and their correlation with arterial elasticity and endothelium-dependent dilation in patients with type 2 diabetes mellitus. Atherosclerosis 2010, 208, 264–269.
- Preston, R.A.; Jy, W.; Jimenez, J.J.; Mauro, L.M.; Horstman, L.L.; Valle, M.; Aime, G.; Ahn, Y.S. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension 2003, 41, 211–217.
- Huang, P.H.; Huang, S.S.; Chen, Y.H.; Lin, C.P.; Chiang, K.H.; Chen, J.S.; Tsai, H.Y.; Lin, F.Y.; Chen, J.W.; Lin, S.J. Increased circulating CD31+/annexin V+ apoptotic microparticles and decreased circulating endothelial progenitor cell levels in hypertensive patients with microalbuminuria. J. Hypertens. 2010, 28, 1655–1665.
- Mostefai, H.A.; Agouni, A.; Carusio, N.; Mastronardi, M.L.; Heymes, C.; Henrion, D.; Andriantsitohaina, R.; Martinez, M.C. Phosphatidylinositol 3-kinase and xanthine oxidase regulate nitric oxide and reactive oxygen species productions by apoptotic lymphocyte microparticles in endothelial cells. J. Immunol. 2008, 180, 5028–5035.
- Soriano, A.O.; Jy, W.; Chirinos, J.A.; Valdivia, M.A.; Velasquez, H.S.; Jimenez, J.J.; Horstman, L.L.; Kett, D.H.; Schein, R.M.; Ahn, Y.S. Levels of endothelial and platelet microparticles and their interactions with leukocytes negatively correlate with organ dysfunction and predict mortality in severe sepsis. Crit. Care Med. 2005, 33, 2540–2546.
- Loyer, X.; Vion, A.C.; Tedgui, A.; Boulanger, C.M. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ. Res. 2014, 114, 345–353.
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005, 11, 367–368.
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006, 20, 661–669.
- Timmers, L.; Lim, S.K.; Arslan, F.; Armstrong, J.S.; Hoefer, I.E.; Doevendans, P.A.; Piek, J.J.; El Oakley, R.M.; Choo, A.; Lee, C.N.; et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007, 1, 129–137.
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.; Timmers, L.; van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312.
- Cantaluppi, V.; Biancone, L.; Figliolini, F.; Beltramo, S.; Medica, D.; Deregibus, M.C.; Galimi, F.; Romagnoli, R.; Salizzoni, M.; Tetta, C.; et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant. 2012, 21, 1305–1320.
- Sahoo, S.; Klychko, E.; Thorne, T.; Misener, S.; Schultz, K.M.; Millay, M.; Ito, A.; Liu, T.; Kamide, C.; Agrawal, H.; et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ. Res. 2011, 109, 724–728.
- Chen, L.; Wang, Y.; Pan, Y.; Zhang, L.; Shen, C.; Qin, G.; Ashraf, M.; Weintraub, N.; Ma, G.; Tang, Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem. Biophys. Res. Commun. 2013, 431, 566–571.
- Deregibus, M.C.; Cantaluppi, V.; Calogero, R.; Lo Iacono, M.; Tetta, C.; Biancone, L.; Bruno, S.; Bussolati, B.; Camussi, G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 2007, 110, 2440–2448.
- Diehl, P.; Fricke, A.; Sander, L.; Stamm, J.; Bassler, N.; Htun, N.; Ziemann, M.; Helbing, T.; El-Osta, A.; Jowett, J.B.; et al. Microparticles: Major transport vehicles for distinct microRNAs in circulation. Cardiovasc. Res. 2012, 93, 633–644.
- Ikeda, S.; Kong, S.W.; Lu, J.; Bisping, E.; Zhang, H.; Allen, P.D.; Golub, T.R.; Pieske, B.; Pu, W.T. Altered microRNA expression in human heart disease. Physiol. Genom. 2007, 31, 367–373.
- Fichtlscherer, S.; De Rosa, S.; Fox, H.; Schwietz, T.; Fischer, A.; Liebetrau, C.; Weber, M.; Hamm, C.W.; Röxe, T.; Müller-Ardogan, M.; et al. Circulating microRNAs in patients with coronary artery disease. Circ. Res. 2010, 107, 677–684.
- Zampetaki, A.; Mayr, M. MicroRNAs in vascular and metabolic disease. Circ. Res. 2012, 110, 508–522.
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 2008, 15, 261–271.
- Jansen, F.; Yang, X.; Hoelscher, M.; Cattelan, A.; Schmitz, T.; Proebsting, S.; Wenzel, D.; Vosen, S.; Franklin, B.S.; Fleischmann, B.K.; et al. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation 2013, 128, 2026–2038.
More
