Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by Jessie Wu and Version 3 by Jessie Wu.
This entry describes a study verified the safety of fermented oat (Avena sativa) when used in a dog food as part of the effort toward discovering suitable nutritionally excellent and functional food materials.
- pet food
- oat
1. Background
Amid increasing global concerns over the shortage of future food resources [1][2], the rapid growth of the pet food market is raising concerns regarding whether a stable supply of raw materials for pet food can be maintained. In addition, hypercompetition in the pet food market has led to the indiscriminate use of new ingredients where preliminary verifications of safety and nutritional value are lacking [3]. In this regard, the need for research on the nutritional value, safety, and functionality of novel ingredients that can replace existing ingredients has been emphasized [4].
In the pet food industry, livestock products are mostly used as a protein source, but the demand for novel protein materials is increasing due to the competitively for their use in food for humans, and regarding the sustainability of livestock products. In other words, it is known that livestock products mainly used as protein sources in dog food can cause allergies. Therefore, many efforts, such as towards using hydrolyzed proteins and finding alternative protein ingredients, have been made to reduce the allergic response induced by exposure to protein sources in dogs [5]. Many researchers are paying attention to the potential suitability of edible insects for human food as well as animal feed [6][7][8]. In particular, black soldier fly larva (BSFL; Hermetia illucens L.) has been reported to be a suitable insect species given its nutritional value, safety, and amenability for mass production [9][10]. Several studies have reported that BSFL meal can partially replace major protein sources (e.g., fish and soybean meal) in conventional diets for poultry [11][12], fish [13][14], and pigs [15]. In addition, some recent studies that evaluated the safety and physiological effects of a BSFL diet on companion dogs have reported positive results [16][17][18]. Nonetheless, more research is needed in terms of the safety and feeding effects of using BSFL in pet food.
Recently, the trend in the pet food market is showing a shift toward grain-free, gluten-free, human-grade, natural, and organic pet food. However, scientific evidence that these are nutritionally superior or that they are more beneficial to pet’s health is lacking. Oats, one of the grains, are known to provide nutrients such as proteins, unsaturated fatty acids, vitamins and minerals, as well as arabinoxylan, β-glucan, and phenolic compounds, having multiple functional and bioactive properties [19][20]. Although there are very limited studies on the efficacy of oat feeding in dogs, one study reported that the intake of oat beta-glucan could improve the apparent total tract digestibility of macronutrients, and was effective in reducing serum total cholesterol and low-density lipoproteins in adult dogs [21]. Meanwhile, although not studied in dogs, previous studies have suggested that oats with various biologically active substances help to prevent diseases, such as cardiovascular pathologies, colon cancer, type II diabetes, and obesity in humans [22][23][24][25][26]. Furthermore, attempts have been devoted to developing oat-based fermented foods using lactic acid bacteria to improve the nutritional value and functionality of oats [27][28][29]; some studies have confirmed the potential value of fermented oats as a functional food [30][31][32]. Despite the positive effects of oats or fermented oats (FOs), no study has reported the effects of feeding FOs to dogs.
2. Food Intake, Body Parameters, and Fecal Score
Table 1 shows the daily food intake, BW, and BCS, for which no significant differences were found among the CON and each treatment group. In all groups, the body weight was higher at the end of the experiment than at the beginning, but there was no significant difference among the control dogs and those fed FO and BSFL.
Table 1. Effects of dog food with FO and BSFL on food intake and body parameters in dogs at the beginning (initial) and end (final) of the experiment.
Values are expressed as mean ± SE. WBC, white blood cell; NEU, neutrophils; LYM, lymphocytes; MONO, monocytes; EOS, eosinophils; BASO, basophils; RBC, red blood cells; HGB, hemoglobin; HCT, hematocrit. *, significant differences from the control in the same row (p < 0.05); #, significant differences between the initial and final values in the same column (p < 0.05). CON, control group; FO, group with 10% fermented oat added to food; BSFL, group with 5% black soldier fly larva added to food; FO + BSFL, group with 10% fermented oat and 5% black soldier fly larva added to food.
| Items | CON 1 | FO | BSFL | FO + BSFL | p-Value |
|---|---|---|---|---|---|
| ADFI 2, g/d | 98.0 ± 9.2 | 100.4 ± 12.0 | 102.3 ± 10.2 | 100.2 ± 12.0 | 0.735 |
| Body weight, kg |
The results of serum biochemical parameters are presented in Table 3. During the experimental period, no significant effect of feeding FO or BSFL was observed on serum glucose (GLU), creatinine (CREA), blood urea nitrogen (BUN), calcium (CA), alanine aminotransferase (ALT), gamma glutamyltransferase (GGT), or albumin/globulin (A/G) ratio values among all experimental groups. However, the FO and FO + BSFL groups showed significantly lower alkaline phosphatase (ALKP) than the control group at the end of the experiment (p < 0.05). For the FO + BSFL group, we recorded significantly lower GLOB compared to the control group at the end of the experiment (p < 0.05). For the BSFL group, we recorded significantly lower phosphorous (PHOS) compared to the control group (p < 0.05). In addition, the BSFL group showed significantly less total protein (T-PRO) and total cholesterol (T-CHO) at the end compared to the beginning of the experiment (p < 0.05).
Table 3. Effects of dog food with FO and BSFL on serum biochemical parameters in dogs.
Values are expressed as mean ± SE. 1 CON, control group; FO, group with 10% fermented oat added to food; BSFL, group with 5% black soldier fly larva added to food; FO + BSFL, group with 10% fermented oat and 5% black soldier fly larva added to food. 2 ADFI, average daily food intake; 3 BWG, body weight gain; 4 BCS, body condition score.
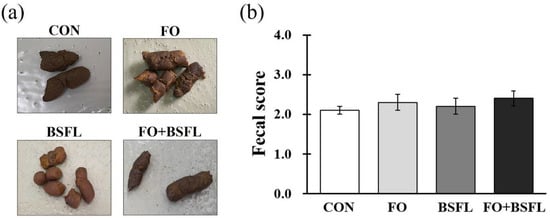
The fecal scores of all the experimental groups were within the desirable range of between 2.10 and 2.40, and the dog food with FO and BSFL did not affect the fecal scores (Figure 1a,b).
| Items | CON | FO | BSFL | FO + BSFL | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.947 | |||||||||||
| GLU, mg/dL (Ref. range: 70–138) | |||||||||||
| Initial | 97.2 ± 8 | 98.6 ± 4.34 | |||||||||
| 103.6 ± 6.45 | 95.8 ± 6.07 | 0.835 | Initial | 4.13 ± 0.75 | 4.22 ± 0.67 | 4.20 ± 0.67 | 4.17 ± 0.67 | 0.999 | |||
| Final | 94.6 ± 4.58 | 95.6 ± 4.34 | 91.8 ± 3.12 | 97 ± 3.51 | 0.816 | Final | 4.49 ± 0.83 | 4.52 ± 0.76 | 4.62 ± 0.76 | 4.51 ± 0.73 | 0.999 |
| Rate of BWG 3, % | |||||||||||
| CREA, mg/dL (Ref. range: 0.5–1.6) | 108.7 ± 1.2 | 106.5 ± 1.2 | 109.8 ± 2.1 | 108.0 ± 1.0 | 0.437 | ||||||
| Initial | 0.64 ± 0.09 | 0.64 ± 0.07 | 0.68 ± 0.05 | BCS 4 | |||||||
| Initial | 4.20 ± 0.73 | 4.20 ± 0.58 | 3.60 ± 0.93 | 3.40 ± 0.87 | 0.850 | ||||||
| Final | 4.60 ± 0.68 | 4.20 ± 0.49 | 3.60 ± 0.81 | 3.40 ± 0.68 | 0.593 | ||||||

Figure 1. Effects of dog food with FO and BSFL on fecal scores of dogs: (a) photographs of the feces; (b) scores based on the following 5-point fecal score scale (1 = hard and dry feces to 5 = liquid diarrhea). The results are expressed as mean ± SE. CON, control group; FO, group with 10% fermented oat added to food; BSFL, group with 5% black soldier fly larva added to food; FO + BSFL, group with 10% fermented oat and 5% black soldier fly larva added to food. The p-value on fecal score was 0.666.
3. Skin Status
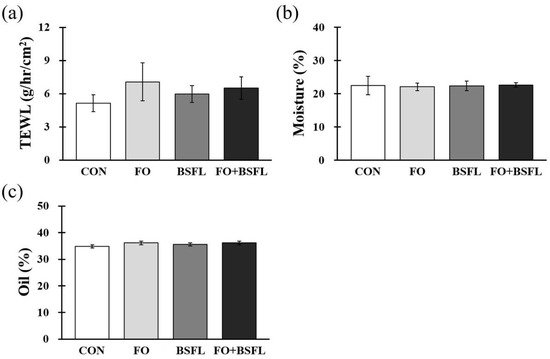
Figure 2 shows the effects of the feeding of FO and BSFL on skin status. At the end of the experiment, the TEWL, moisture, and oil levels of the groin, armpits, back, and ears were measured, and the measured value is expressed as the average of values determined at the four sites. The TEWL, moisture, and oil levels of each treatment group did not show significant differences compared to the control group (Figure 2a–c).
Figure 2. Effects of dog food with FO and BSFL on skin barrier status in dogs. (a) TEWL, (b) moisture, and (c) oil concentration were measured from four different areas (groin skin, armpit skin, back skin, and ear skin) at the end of the experiment. The results are expressed as mean ± SE. TEWL, transepidermal water loss. CON, control group; FO, group with 10% fermented oat added to food; BSFL, group with 5% black soldier fly larva added to food; FO + BSFL, group with 10% fermented oat and 5% black soldier fly larva added to food. p-value: (a) 0.486, (b) 0.565, (c) 0.639.
4. Hematological and Biochemical Parameters
The results of hematological parameters are presented in Table 3. All hematological parameters analyzed in this study were within the normal reference range, and no significant differences in these parameters were observed by the single or combined feeding of BSFL and FO among all experimental groups, except for white blood cells (WBCs) in the BSFL group. At the end of the experiment, the BSFL group had a significantly higher WBC value than the CON group (p < 0.05). Basophils (BASO) were not affected by the feeding FO or BSFL, but BASO in the BSFL group was significantly increased at the end of experiment compared to the beginning (p < 0.05). All the experimental groups showed no significant effects of FO and BSFL on neutrophils (NEU), lymphocytes (LYM), monocytes (MONO), red blood cells (RBC), hemoglobin (HGB), or hematocrit (HCT) during the study period.
Table 2. Effects of dog food with FO and BSFL on CBCs in dogs.
Values are expressed as mean ± SE. GLU, glucose; CREA, creatinine; BUN, blood urea nitrogen; PHOS, phosphorous; CA, calcium; T-Pro, total protein; ALB, albumin; GLOB, globulin; A/G, albumin/globulin ratio; ALT, alanine aminotransferase; ALKP, alkaline phosphatase; GGT, gamma glutamyltransferase; T-BIL, total bilirubin; T-CHO, total cholesterol. *, significant difference from the control in the same row (p < 0.05); #, significant differences between initial and final values in the same column (p < 0.05). CON, control group; FO, group with 10% fermented oat added to food; BSFL, group with 5% black soldier fly larva added to food; FO + BSFL, group with 10% fermented oat and 5% black soldier fly larva added to food.
| Items | CON | FO | BSFL | FO + BSFL | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC, ×106/mL (Ref. range: 5.05–16.76) | ||||||||||||||||
| Initial | 8.08 ± 0.80 | 8.10 ± 1.89 | 10.26 ± 2.15 | 6.28 ± 1.00 | 0.385 | |||||||||||
| Final | 7.48 ± 0.81 | 9.48 ± 2.88 | 12.24 ± 1.10 * | 7.65 ± 1.08 | 0.194 | |||||||||||
| NEU, ×106/mL (Ref. range: 2.95–11.64) | ||||||||||||||||
| Initial | 5.42 ± 0.53 | 4.97 ± 1.23 | 7.23 ± 1.71 | 4.11 ± 0.86 | 0.321 | |||||||||||
| Final | 5.58 ± 0.54 | 6.56 ± 2.21 | 8.38 ± 1.16 | 5.57 ± 0.64 | 0.415 | |||||||||||
| LYM, ×106/mL (Ref. range: 1.05–5.10) | ||||||||||||||||
| Initial | 1.69 ± 0.28 | 2.15 ± 0.38 | 1.82 ± 0.29 | 1.40 ± 0.16 | 0.362 | |||||||||||
| Final | 1.48 ± 0.26 | 2.06 ± 0.39 | 2.74 ± 0.79 | 1.34 ± 0.30 | 0.200 | |||||||||||
| MONO, ×106/mL (Ref. range: 0.16–1.12) | ||||||||||||||||
| Initial | ||||||||||||||||
| Final | 0.55 ± 0.24 | |||||||||||||||
| HCT, % (Ref. range: 37.3–61.7) | ||||||||||||||||
| 0.6 ± 0.05 | 0.883 | |||||||||||||||
| Initial | ||||||||||||||||
| 41.28 ± 1.65 | ||||||||||||||||
| Final | 0.72 ± 0.12 | 0.72 ± 0.09 | 0.72 ± 0.07 | 0.68 ± 0.0714.6 ± 1.25 | 15 ± 1.22 | |||||||||||
| 40.36 ± 1.03 | ||||||||||||||||
| 42.24 ± 1.06 | 40.52 ± 1.76 | 0.777 | ||||||||||||||
| 0.984 | ||||||||||||||||
| BUN, mg/dL (Ref. range: 6.0–31) | ||||||||||||||||
| Initial | 13.6 ± 0.93 | 14.4 ± 1.63 | 16.4 ± 1.47 | 15 ± 1.38 | 0.54515.8 ± 1.2 | 14 ± 1.41 | 0.789 | |||||||||
| PHOS, mg/dL (Ref. range: 2.5–6.0) | 0.65 ± 0.25 | 0.65 ± 0.25 | 0.46 ± 0.13 | 0.919 | ||||||||||||
| Initial | 4.74 ± 0.35 | 4.62 ± 0.37 | 4.22 ± 0.29 | 4.32 ± 0.32 | 0.655 | Final | 0.19 ± 0.12 | 0.55 ± 0.29 | 0.74 ± 0.20 | 0.53 ± 0.18 | 0.324 | |||||
| Final | 4.54 ± 0.26 | 4.54 ± 0.47 | 3.52 ± 0.31 * | 3.72 ± 0.25 | 0.049 | EOS, ×106/mL (Ref. range: 0.06–1.23) | ||||||||||
| CA, mg/dL (Ref. range: 8.9–11.4) | Initial | 0.43 ± 0.07 | 0.33 ± 0.10 | 0.56 ± 0.22 | 0.31 ± 0.08 | 0.542 | ||||||||||
| Initial | 9.18 ± 0.41 | 9.34 ± 0.29 | 9.64 ± 0.48 | 9.64 ± 0.21 | 0.295 | Final | 0.06 ± 0.01 | 0.16 ± 0.05 | 0.27 ± 0.15 | 0.14 ± 0.06 | 0.406 | |||||
| Final | 9.08 ± 0.36 | 9.38 ± 0.16 | 8.96 ± 0.32 | 8.9 ± 0.23 | 0.628 | BASO, ×106/mL (Ref. range: 0–0.1) | ||||||||||
| T-Pro, g/dL (Ref. range: 5.0–7.4) | Initial | 0.00 ± 0.00 | 0.002 ± 0.00 | 0.004 ± 0.00 | 0.00 ± 0.00 | 0.547 | ||||||||||
| Initial | 6.98 ± 0.37 | 6.76 ± 0.27 | 7.24 ± 0.17 | 6.24 ± 0.2 | 0.089 | Final | 0.05 ± 0.02 | 0.11 ± 0.05 | 0.10 ± 0.03 # | 0.07 ± 0.03 | 0.516 | |||||
| RBC, ×10 | ||||||||||||||||
| Final | 7.34 ± 0.26 | 6.66 ± 0.25 | 6.62 ± 0.19 # | 6.56 ± 0.22 | 0.100 | 9/mL (Ref. range: 5.65–8.87) | ||||||||||
| Initial | 5.85 ± 0.26 | 5.84 ± 0.17 | 6.11 ± 0.09 | 5.88 ± 0.27 | 0.783 | |||||||||||
| Final | 6.27 ± 0.31 | 6.01 ± 0.11 | 6.33 ± 0.18 | 6.02 ± 0.31 | 0.702 | |||||||||||
| HGB, g/dL (Ref. range: 13.1–20.5) | ||||||||||||||||
| Initial | 14.26 ± 0.61 | |||||||||||||||
| ALB, g/dL (Ref. range: 2.7–4.4) | ||||||||||||||||
| Initial | 3.16 ± 0.12 | 3.22 ± 0.08 | 3.44 ± 0.11 | 2.94 ± 0.14 | 0.087 | |||||||||||
| Final | 3.22 ± 0.09 | 3.2 ± 0.09 | 3.14 ± 0.12 | 3.14 ± 0.1313.94 ± 0.47 | 14.32 ± 0.44 | 14.16 ± 0.64 | 0.963 | |||||||||
| 0.935 | ||||||||||||||||
| GLOB, g/dL (Ref. range: 1.6–3.6) | Initial | 3.82 ± 0.28 | 3.54 ± 0.22 | 3.8 ± 0.17 | 3.3 ± 0.13 | 0.271 | Final | 14.40 ± 0.69 | 13.82 ± 0.54 | 14.14 ± 0.41 | 14.18 ± 0.99 | |||||
| Final | 4.12 ± 0.23 | 3.46 ± 0.29 | 3.48 ± 0.19 | 3.42 ± 0.12 * | 0.048 | |||||||||||
| A/G ratio (Ref. range: 0.8–2.0) | ||||||||||||||||
| Initial | 0.84 ± 0.05 | 0.92 ± 0.05 | Final | 44.51 ± 1.98 | 42.27 ± 0.88 | 43.89 ± 0.93 | 43.16 ± 2.25 | 0.790 | ||||||||
| 0.92 ± 0.07 | |||||
| 0.88 ± 0.06 | |||||
| 0.715 | |||||
| Final | |||||
| 0.78 ± 0.06 | |||||
| 0.98 ± 0.09 | |||||
| 0.92 ± 0.07 | |||||
| 0.9 ± 0.03 | |||||
| 0.221 | |||||
| ALT, U/L (Ref. range: 12–118) | |||||
| Initial | |||||
| 111.4 ± 48.17 | 58.8 ± 13.75 | 135.5 ± 43.56 | 41.2 ± 6.18 | 0.164 | |
| Final | 87 ± 31.58 | 38.4 ± 9.9 | 97.5 ± 35.26 | 35.4 ± 8.07 | 0.246 |
| ALKP, U/L (Ref. range: 5.0–131) | |||||
| Initial | 60.6 ± 19.94 | 24.6 ± 3.61 | 62 ± 17.52 | 20.6 ± 4.55 | 0.079 |
| Final | 58.4 ± 7.93 | 36.4 ± 4.17 * | 70 ± 24.03 | 29 ± 5.39 * | 0.043 |
| GGT, U/L (Ref. range: 0–12) | |||||
| Initial | 0 ± 0 | 0 ± 0 | 3 ± 3 | 0 ± 0 | 0.418 |
| Final | 0 ± 0 | 0 ± 0 | 2.8 ± 2.8 | 0 ± 0 | 0.418 |
| T-BIL, mg/dL (Ref. range: 0.1–0.3) | |||||
| Initial | 0.28 ± 0.04 | 0.22 ± 0.04 | 0.4 ± 0.03 * | 0.3 ± 0.03 | 0.004 |
| Final | 0.28 ± 0.04 | 0.2 ± 0.04 | 0.3 ± 0.05 | 0.2 ± 0.03 | 0.254 |
| CHOL, mg/dL (Ref. range: 29–291) | |||||
| Initial | 131.4 ± 15.16 | 136.4 ± 8.89 | 177.6 ± 8.95 * | 132.8 ± 6.58 | 0.017 |
| Final | 143.2 ± 18.99 | 141.8 ± 10.94 | 159.4 ± 9.89 | 153 ± 10.29 | 0.749 |
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32]The values for all the parameters of the FO and FO + BSFL groups remained within the reference ranges throughout the experiment. However, the control group showed slightly higher GLOB values both at the beginning (3.82 ± 0.28 g/dL) and at the end of the experiment (4.12 ± 0.23 g/dL) compared to the reference range (1.6–3.6 g/dL). Although the BSFL group showed slightly higher GLOB (3.80 ± 0.17 g/dL) and T-BIL (0.40 ± 0.03 mg/dL) values compared to the reference ranges (GLOB, 1.6–3.6 g/dL; T-BIL, 0.1–0.3 g/dL) at the beginning of the experiment, they were measured as being within the normal ranges at the end of the experiment (Table 3).
5. IgG and Cytokines
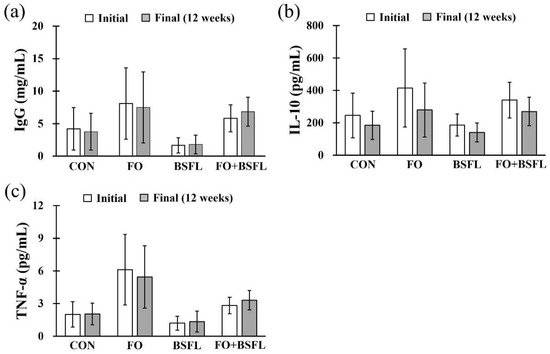
Figure 3 shows the effects of feeding FO and BSFL on changes in canine immunoglobulin G (IgG), interleukin 10 (IL-10), and tumor necrosis factor alpha (TNF-α) levels. Canine IgG ranged between 1.68 and 8.10 mg/mL, the IL-10 ranged between 140.24 and 415.58 pg/mL, and the TNF-α between 1.20 and 6.12 pg/mL, and no statistically significant differences were found in any of the experimental groups compared to the control group. In addition, no significant changes were observed in the levels of IgG, IL-10, or TNF-α in any experimental group for 12 weeks (initial vs. final; Figure 3).
Figure 3. Effect of dog food with FO and BSFL on the IgG and inflammatory cytokines in dogs: (a) IgG, (b) IL-10, and (c) TNF-α. The results are expressed as mean ± SE. IgG, immunoglobulin G; IL-10, interleukin 10; TNF-α, tumor necrosis factor alpha. CON, control group; FO, group with 10% fermented oat added to food; BSFL, group with 5% black soldier fly larva added to food; FO + BSFL, group with 10% fermented oat and 5% black soldier fly larva added to food.
Comprehensively, the feeding of 10% FO and 5% BSFL for 12 weeks did not affect food intake, body weight, or BCS, and did not have a negative effect on physiological and biochemical responses in dogs. Furthermore, the findings suggest that BSFL may have the ability to reduce serum total cholesterol in dogs. Further studies of the effects of BSFL on serum total cholesterol in dogs are required. The results demonstrate the safety and potential functionality of FO and BSFL, and verify their suitability as food ingredients for dogs.
References
- Food and Agriculture Organization of the United Nations (FAO). High-Level Expert Forum—How to Feed the World in 2050; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2009.
- Boland, M.J.; Rae, A.N.; Vereijken, J.M.; Meuwissen, M.P.; Fischer, A.R.; van Boekel, M.A.; Rutherfurd, S.M.; Gruppen, H.; Moughan, P.J.; Hendriks, W.H. The future supply of animal-derived protein for human consumption. Trends Food Sci. Technol. 2013, 29, 62–73.
- Alvarenga, C.I.; Aldrich, C.G. The effect of increasing levels of dehulled faba beans (Vicia faba L.) on extrusion and product parameters for dry expanded dog food. Foods 2019, 8, 26.
- McCusker, S.; Buff, P.R.; Yu, Z.; Fascetti, A.J. Amino acid content of selected plant, algae and insect species: A search for alternative protein sources for use in pet foods. J. Nutr. Sci. 2014, 3, e39.
- Verlinden, A.; Hesta, M.; Millet, S.; Janssens, G.P.J. Food allergy in dogs and cats: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 259–273.
- Nadeau, L.; Nadeau, I.; Franklin, F.; Dunkel, F. The potential for entomophagy to address undernutrition. Ecol. Food Nutr. 2014, 54, 200–208.
- Verneau, F.; Amato, M.; La Barbera, F. Edible insects and global food security. Insects 2021, 12, 472.
- Areerat, S.; Chundang, P.; Lekcharoensuk, C.; Kovitvadhi, A. Possibility of using house cricket (Acheta domesticus) or mulberry silkworm (Bombyx mori) pupae meal to replace poultry meal in canine diets based on health and nutrient digestibility. Animals 2021, 11, 2680.
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600.
- Wang, Y.-S.; Shelomi, M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 2017, 6, 91.
- Onsongo, V.O.; Osuga, I.M.; Gachuiri, C.K.; Wachira, A.M.; Miano, D.M.; Tanga, C.M.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Insects for income generation through animal feed: Effect of dietary replacement of soybean and fish meal with black soldier fly meal on broiler growth and economic performance. J. Econ. Èntomol. 2018, 111, 1966–1973.
- Marono, S.; Loponte, R.; Lombardi, P.; Vassalotti, G.; Pero, M.E.; Russo, F.; Gasco, L.; Parisi, G.; Piccolo, G.; Nizza, S.; et al. Productive performance and blood profiles of laying hens fed Hermetia illucens larvae meal as total replacement of soybean meal from 24 to 45 weeks of age. Poult. Sci. 2017, 96, 1783–1790.
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 2017, 8, 57.
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbo, R.; Krogdahl, Å.; Lock, E.-J. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 2019, 503, 609–619.
- Chia, S.; Tanga, C.; Osuga, I.; Alaru, A.; Mwangi, D.; Githinji, M.; Dubois, T.; Ekesi, S.; van Loon, J.; Dicke, M. Black soldier fly larval meal in feed enhances growth performance, carcass yield and meat quality of finishing pigs. J. Insects Food Feed. 2021, 7, 433–447.
- Lei, X.; Kim, T.; Park, J.; Kim, I. Evaluation of Supplementation of Defatted Black Soldier Fly (Hermetia illucens) Larvae Meal in Beagle Dogs. Ann. Anim. Sci. 2019, 19, 767–777.
- Kröger, S.; Heide, C.; Zentek, J. Evaluation of an extruded diet for adult dogs containing larvae meal from the Black soldier fly (Hermetia illucens). Anim. Feed. Sci. Technol. 2020, 270, 114699.
- Freel, T.A.; McComb, A.; Koutsos, E.A. Digestibility and safety of dry black soldier fly larvae meal and black soldier fly larvae oil in dogs. J. Anim. Sci. 2021, 99, 47.
- Menon, R.; Gonzalez, T.; Ferruzzi, M.; Jackson, E.; Winderl, D.; Watson, J. Oats-from farm to fork. Adv. Food Nutr. Res. 2016, 77, 1–55.
- Soycan, G.; Schär, M.Y.; Kristek, A.; Boberska, J.; Alsharif, S.N.; Corona, G.; Shewry, P.R.; Spencer, J.P. Composition and content of phenolic acids and avenanthramides in commercial oat products: Are oats an important polyphenol source for consumers? Food Chem. X 2019, 3, 100047.
- Ferreira, L.G.; Endrighi, M.; Lisenko, K.G.; Oliveira, M.R.D.; Damasceno, M.R.; Claudino, J.A.; Gutierres, P.G.; Peconick, A.P.; Saad, F.M.O.B.; Zangeronimo, M.G. Oat beta-glucan as a dietary supplement for dogs. PLoS ONE 2018, 13, e0201133.
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313.
- Whitehead, A.; Beck, E.J.; Tosh, S.; Wolever, T.M. Cholesterol-lowering effects of oat β-glucan: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421.
- Bao, L.; Cai, X.; Xu, M.; Li, Y. Effect of oat intake on glycaemic control and insulin sensitivity: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2014, 112, 457–466.
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Mejia, S.B.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The effect of oatβ-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomised-controlled trials. Br. J. Nutr. 2016, 116, 1369–1382.
- Chang, H.-C.; Huang, C.-N.; Yeh, D.-M.; Wang, S.-J.; Peng, C.-H.; Wang, C.-J. Oat prevents obesity and abdominal fat distribution, and improves liver function in humans. Plant Foods Hum. Nutr. 2013, 68, 18–23.
- Angelov, A.; Gotcheva, V.; Kuncheva, R.; Hristozova, T. Development of a new oat-based probiotic drink. Int. J. Food Microbiol. 2006, 112, 75–80.
- Gupta, S.; Abu-Ghannam, N. Probiotic fermentation of plant based products: Possibilities and opportunities. Crit. Rev. Food Sci. Nutr. 2012, 52, 183–199.
- Asadzadeh, A.; Jalali, H.; Azizi, M.H.; Nafchi, A.M. Production of oat bran functional probiotic beverage using Bifidobacterium lactis. J. Food Meas. Charact. 2021, 15, 1301–1309.
- Russo, P.; de Chiara, M.L.V.; Capozzi, V.; Arena, M.P.; Amodio, M.L.; Rascón, A.; Dueñas, M.T.; López, P.; Spano, G. Lactobacillus plantarum strains for multifunctional oat-based foods. LWT. 2016, 68, 288–294.
- Bei, Q.; Wu, Z.; Chen, G. Dynamic changes in the phenolic composition and antioxidant activity of oats during simultaneous hydrolysis and fermentation. Food Chem. 2020, 305, 125269.
- Bocchi, S.; Rocchetti, G.; Elli, M.; Lucini, L.; Lim, C.-Y.; Morelli, L. The combined effect of fermentation of lactic acid bacteria and in vitro digestion on metabolomic and oligosaccharide profile of oat beverage. Food Res. Int. 2021, 142, 110216.
More
