Pathogenic filamentous fungi are the most important causal agents of postharvest decay of fresh fruits. Depending on the fruit species, cultivar, and a wide range of pre- and postharvest factors and conditions, the incidence of fungal decay can cause considerable economic losses to growers and traders, especially if the produce is intended for export markets. Control of postharvest diseases of fresh fruits has relied for many years on the continuous use of conventional chemical fungicides. However, nonpolluting alternatives are increasingly needed because of human health and environmental issues related to the generation of chemical residues. Low-toxicity chemicals classified as food preservatives or as generally recognized as safe (GRAS) compounds have known and very low toxicological effects on mammals and minimal impact on the environment. Among them, inorganic or organic salts such as carbonates, sorbates, benzoates, silicates, etc., show significant advantages for potential commercial use, such as their availability, low cost, and general high solubility in water.
1. Introduction
Pathogenic filamentous fungi are the most important causal agents of postharvest decay of fresh fruits. Depending on the fruit species, cultivar, and a wide range of pre- and postharvest factors and conditions, the incidence of fungal decay can cause considerable economic losses to growers and traders, especially if the produce is intended for export markets. Wholesale buyers often reject fruit loads if decay is found in export shipments, and furthermore, they may charge the producer for the transport and handling costs
[1].
Every fresh fruit commodity is prone to decay caused by pathogenic fungi. Depending on the origin and characteristics of the infection, fungi that cause postharvest diseases can be classified into two general groups: (i) those that infect the fruit in the field and remain latent until their development after harvest, and (ii) those that infect the fruit through rind microwounds or injuries inflicted during harvest, transportation, postharvest handling, and commercialization
[2]. Typically, fungal species that cause latent infections can also cause wound infections near or after harvest in the same fruit commodity under certain conditions, but the opposite is not true, and many economically important wound pathogens can only infect fruit if its peel is broken, therefore they are known as strict wound pathogens. This is the case of
Penicillium spp., the causal agents of blue or green molds on many relevant fruit commodities.
For example, blue mold, caused by the species
Penicillium expansum L., can lead to significant postharvest losses of apple, pear, stone fruits, kiwifruit, many berries, pomegranate, persimmon, and other subtropical and tropical fruits
[3][4][5]. The two postharvest diseases that account for the largest decay losses of citrus fruits worldwide are green and blue molds, caused by
Penicillium digitatum (Pers.:Fr.) Sacc. and
Penicillium italicum Wehmer, respectively
[6]. It is clear from these denominations that the common names of postharvest diseases are based on the symptoms they produce. Other important wound pathogens are
Geotrichum spp., which cause sour rot on different horticultural products, and
Rhizopus spp., which are dangerous postharvest pathogens that cause soft rot or Rhizopus rot on a wide range of fruit hosts. The most important species are
Geotrichum candidum L., which attacks stone fruits such as peach, nectarine, plum, and sweet cherry, but also tomato and other fruit-like vegetables; and
Geotrichum citri-aurantii (Ferraris) Butler, the cause of sour rot of citrus fruits.
Rhizopus stolonifer (Ehrenb.) Vuill. is the most important species causing fruit soft rot
[7][8][9].
Among latent pathogens,
Botrytis cinerea Pers.:Fr., which causes gray mold, is the main causal agent of postharvest decay of grape, strawberry, blueberry and other small berries, kiwifruit, pomegranate, fig, etc., but also attacks many other fruits and vegetables
[10][11].
Colletotrichum spp. cause anthracnose on many subtropical and tropical fruits, such as citrus fruit, avocado, persimmon, banana, papaya, mango, and pineapple. The most important species are
C. gloeosporioides (Penz.) Penz. & Sacc.,
C. acutatum J.H. Simmonds, and
C. horii B.S. Weir & P.R. Johnst.
[12][13].
Alternaria spp., which causes black spot, and
Lasiodiplodia theobromae (Pat.) Griffon and Maubl. and other Botryosphaeriaceae pathogens, which cause stem-end rot, are also economically relevant for the worldwide industry of subtropical and tropical fruit crops
[14][15]. The species
Alternaria alternata (Fr.) Keissl. is also an important postharvest pathogen of pome fruits, persimmon, loquat, and pomegranate, among many other fruits
[16]. Species of
Monilinia, especially
M. fructicola (Winter) Honey,
M. laxa (Aderhold & Ruhland) Honey, and
M. fructigena (Aderhold & Ruhland) Honey, cause field and postharvest brown rot of stone fruits and are generally considered among the most substantial limiting factors of the yield of these fruit crops
[17].
Monilinia spp. can also cause postharvest disease on pome fruits
[18].
For many years now, fruit postharvest diseases and the economic losses they cause have been contained worldwide through postharvest applications of synthetic chemical fungicides in commercial packinghouses. Active ingredients such as imazalil (IMZ), pyrimethanil (PYR), fludioxonil (FLU), thiabendazole (TBZ), and others are being extensively used as cost-effective means of postharvest decay control in conventional horticulture. However, the massive and continuous use of these chemicals is increasingly leading to significant problems, such as human health issues and environmental contamination due to chemical residues, reduced efficacy of many synthetic fungicides due to the proliferation of resistant fungal biotypes, and restricted access to new high-value organic markets or traditional export markets that are now demanding products with lower levels of pesticides to satisfy consumer demands. Therefore, consumer trends and legislative updates clearly favor a reduction in the use of conventional fungicides, which makes it necessary to potentiate research to develop and implement alternative approaches and novel technologies for the control of postharvest diseases. If conventional chemicals are not available, effective control will need to adopt integrated strategies in which, besides new nonpolluting postharvest antifungal treatments, all factors affecting disease epidemiology and incidence will need to be taken into account, including preharvest factors
[19][20].
2. Evaluation and Selection of GRAS Salts
Although the acidic forms of some GRAS salts can also show substantial antimicrobial activity, salt compounds are preferred as potential postharvest treatments because of their superior solubility and ease of manipulation and application. Moreover, the additional antifungal activity against important postharvest pathogens of cations such as Na+, K+, and NH4+ has been proven for many salts
[21][22].
Research with GRAS salts to control fruit postharvest decay generally implies a sequential procedure.
In vitro tests are useful to assess the toxicity of different salt concentrations to the target postharvest pathogen. Aqueous solutions of selected salts at selected concentrations can then be used in
in vivo laboratory tests, with fruit artificially inoculated with pathogens to determine the control ability of the salt in conditions that resemble potential applications in the packinghouse. The commercial value of the potential implementation of postharvest treatments with selected salt solutions at selected concentrations can be tested afterwards in semicommercial or commercial trials. More recently, interest has grown with regard to the use of antifungal GRAS salts as ingredients of edible coatings. In the fresh fruit industry, such coatings are devoted for increased storage life, but also as a substitute for the prestorage waxes that are often applied in mixed formulations with conventional fungicides.
2.1. In Vitro Antifungal Activity
Given a postharvest disease of economic importance, its causal microorganism should be isolated from diseased fruit, purified, properly identified, and multiplied to be used in the experiments. Postharvest fungal pathogens are typically cultured and multiplied on Petri dishes containing potato dextrose agar (PDA) culture medium, although other media such as malt extract agar (MEA), V-8, or dichloran rose-bengal chloramphenicol agar (RBDC) may be used. Commonly, different strains of the same pathogen are isolated from infected fresh fruits found in commercial packinghouses in the commodity producing area, and preliminary
in vivo tests are conducted to select, based on their aggressiveness and uniform behavior, those strains of each species more suitable for use in research. Appropriate incubation temperatures for growth of the most common postharvest pathogens of fresh fruit are between 20 and 25 °C. Depending on the growth rate of the fungal species, the incubation time needed to obtain mature fungal inoculum for use in experiments is 1–3 weeks.
The most typical procedure for
in vitro evaluation of the antifungal activity of GRAS salts is to assess the inhibition of the radial mycelial growth of the target pathogen. For this, the fungus is inoculated on 90 mm diameter plastic Petri dishes with PDA medium amended with the test salt at the desired concentration. Since the solubility in water of most GRAS salts is very high, sterile stock solutions of each salt at high concentration (5–10%) are prepared and serial dilutions are performed to achieve the range of final concentrations that will be tested, frequently from 0.1% or lower to a maximum of 2–3%, depending on the salt characteristics and the regulations established for each of them. These solutions are then incorporated into the autoclaved PDA medium at 40–50 °C, poured into the Petri dishes, and allowed to solidify, all under strict sterile conditions. Spores at a known concentration or, more commonly, mycelial plugs (around 5 mm in diameter) from the edge of the pathogen growing cultures, produced with a sterilized cork borer, are then inoculated at the center of each dish. PDA plates without salt serve as negative controls. Inoculated plates are then incubated in a growth cabinet at 20–25 °C for a period of time that depends on the fungal species, but at least until fungal growth completely covers the control plates. Radial mycelial growth is periodically (every 1, 2, or 3 days) determined in each plate by measuring two perpendicular fungal colony diameters during the entire incubation period. Usually, 3–5 replicate plates are used for each salt and salt concentration. The results are expressed as a percentage of mycelial growth inhibition according to the formula (dc − dt)/dc × 100, where dc is the average diameter of the fungal colony on control plates and dt is the average diameter of the fungal colony on treated (salt-amended) plates. The obtained data are typically subjected to a two-way analysis of variance (ANOVA) with salt and salt concentration as factors. With particular salts selected for their high antifungal activity, it can be useful to establish the minimum inhibitory concentration (MIC) of the salt, which implies testing a larger number of concentrations.
Other valuable information that can be obtained in
in vitro tests is the ability of GRAS salts to kill or inactivate the spores of the target fungal pathogen. For this, typical
in vitro spore mortality or spore germination tests consist of preparing liquid culture medium (PDA broth or similar) containing different concentrations of the GRAS salt, to which aliquots of a spore suspension of known density (usually 10
4–10
6 spores/mL) are aseptically transferred
[22][23]. After 18–24 h of incubation at 20–25 °C, acid fuchsin solution is added to stop further germination and the percentage of germinated spores is determined by observing 100–150 spores with an inverted compound microscope with a micrometer. As a control, the same amount of spore suspension is added to medium broth without GRAS salt. A spore is scored as germinated if the germ tube length is equal to or exceeds that of the spore itself. The data are generally expressed as percent spore germination inhibition and calculated with a formula similar to that described above for the percent mycelial growth inhibition. Each treatment (each salt at a defined concentration) is applied to 3–5 replicates. Microwell plates are often used for this type of test. With particular salts selected for their high antifungal activity, it can be useful to determine the ED
50 or ED
95 values, i.e., the effective doses (salt concentrations) that kill or inactivate 50% or 95%, respectively, of the spores.
2.2. Control Ability of Aqueous Solutions
GRAS salts and concentrations that show significant antifungal activity in
in vitro tests are selected for evaluation of their ability to control disease in
in vivo tests, i.e., in the fruit host. The commercial potential of postharvest treatment with aqueous solutions of salts with antifungal properties is high, because their use in fresh produce packinghouses to substitute for conventional fungicides will not require substantial changes in the mode of application and in the industrial equipment used. Like fungicides, they could be applied in drenchers or in the packing line as dips, sprays, or low-pressure floods.
Different types of
in vivo tests can be designed, depending on the objective and scale of each particular research step. Sequentially, it is common to start with
in vivo primary screenings in the laboratory, continue with small-scale trials, and finish with semicommercial or commercial trials
[24]. The scale and the amount of fruit employed in each of these types of experiments are larger than in the previous one. Fruits are usually collected from commercial orchards or from the packinghouse if no postharvest treatments have been applied yet. They are used in the experiments the same day or following days, or, depending on the commodity, they could be used after a variable, but not prolonged, period of cold storage. It is important before each experiment to properly select, randomize, wash and disinfect, and thoroughly rinse the fruit.
2.3. Performance of Ingredients of Edible Coatings
Another application of antifungal GRAS salts that is increasingly gaining importance is as ingredients of edible coatings for fresh fruit. Postharvest application of these coatings could replace the use of fungicide-amended commercial waxes applied to many fruit commodities and could be used for organic or “zero-residue” fresh fruit production systems.
Waxes and coatings in general are primarily applied to fresh fruits to increase their postharvest life by regulating the exchange of water and gases (oxygen and carbon dioxide). This primary function allows for less weight loss during storage and, in some cases, the alleviation of some postharvest physiological disorders such as chilling injury or rind breakdown. In addition, coatings can also provide shine and gloss to improve the fruit’s external appearance
[25]. Besides natural coatings such as chitosan and
Aloe spp. gels, most coatings are synthetic formulations comprising blends of hydrocolloids (proteins or polysaccharides) and lipids (waxes, acylglycerols, or fatty acids) as constituents of the composite coating matrix. These main ingredients are typically formulated with plasticizers (e.g., sucrose, glycerol, sorbitol, propylene glycol, etc.) and emulsifiers (e.g., fatty acids, polysorbates, monostearates, lecithin, etc.) to enhance the coating integrity and emulsion stability, respectively. Resins such as shellac are also often added to provide gloss
[26][27]. Furthermore, additional ingredients can be incorporated to increase the functionality of these synthetic coatings. Among them, antimicrobial agents, such as some GRAS salts, can be added to prevent or reduce decay during storage
[2][26].
The performance of antifungal edible coatings should be evaluated considering the interactions among all the elements of the system, i.e., the target pathogen, the coating ingredients, the antifungal GRAS salt, and the commodity and its postharvest handling. Therefore, developing these coatings requires initial optimization of coating formulations based on the chemical compatibility of the ingredients to achieve stable emulsions capable of forming homogeneous coatings. For a particular fruit commodity, the matrix and the non-antifungal ingredients of the coating are selected according to the coating’s physiological activity in terms of reducing weight loss and increasing storability. For a particular pathosystem, GRAS salts and concentrations are initially selected in accordance with the control ability of aqueous solutions, hence it is important that previous information on these aspects is available. Coating formulation is usually optimized on the basis of percent total solid content, total lipid content, and GRAS salt concentration, but other parameters such as viscosity, pH, and wettability for the particular fruit commodity are also important
[28]. Formulations that are stable after incorporation of the selected salt at the selected concentration in the selected coating will then be tested to determine their ability to control the target postharvest disease. Incompatible emulsions solidify or show phase separation or undesirable physical characteristics. It can happen that a particular salt is not compatible at all with a particular coating, or that it is compatible only at salt concentrations or coating total solid contents below a specific threshold. For example, among 470 emulsions formulated with a hydroxypropyl methylcellulose (HPMC)-lipid (beeswax and shellac) matrix and about 30 antifungal food additives (mostly GRAS salts) and mixtures at a large range of concentrations, only 25 emulsions were selected for their high stability. They contained 6 to 8% solid content, 50% (dry basis) total lipid content, and a maximum of 2.5% (wet basis) food preservative
[28].
Although the antifungal activity of stable coatings with GRAS salts may be tested
in vitro (disk diameter tests) by preparing dry disks of coating film and placing them on the surface of culture medium previously inoculated with spores of the target pathogen, it is more practical and usual to evaluate the control ability of the coatings in
in vivo tests.
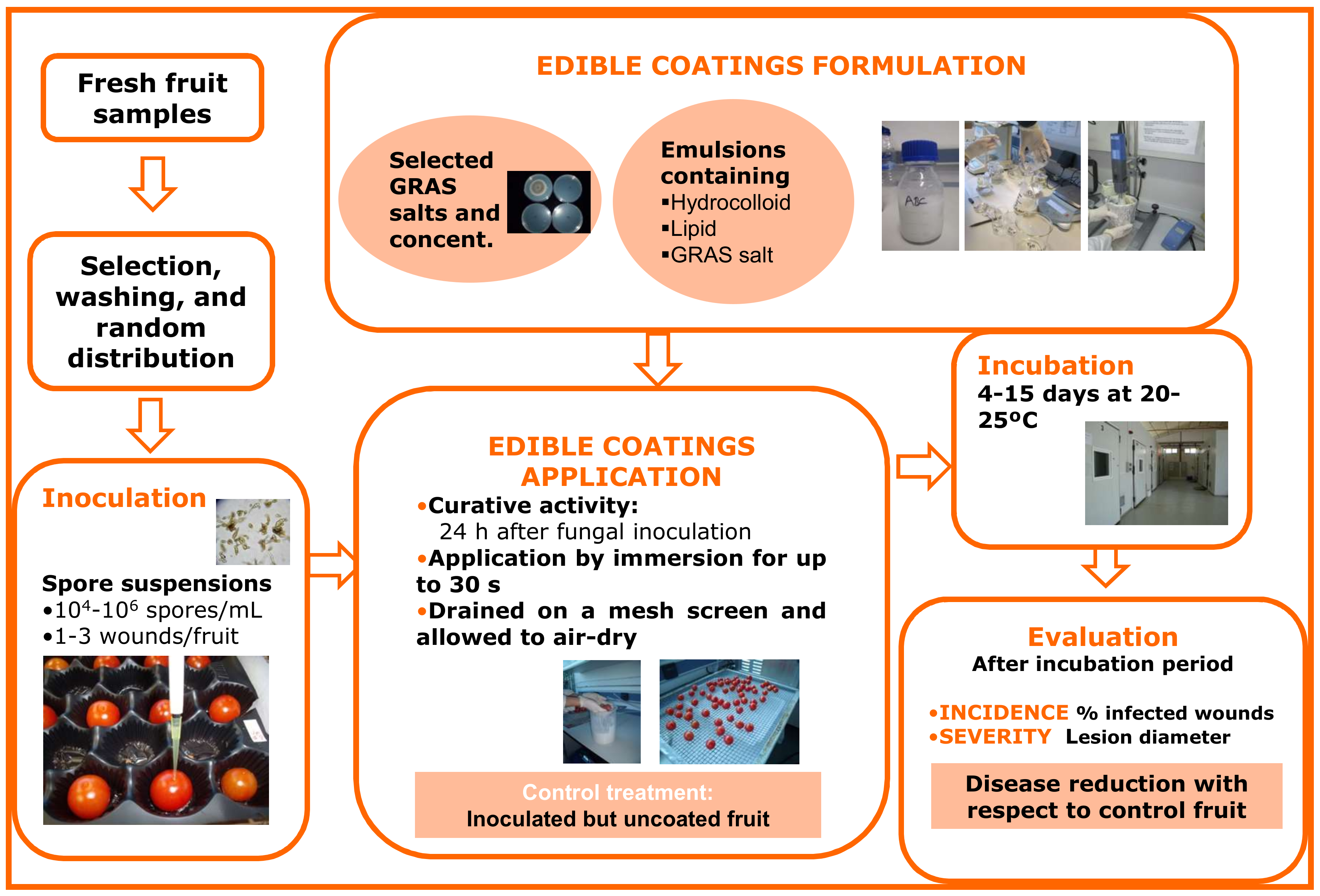
Figure 1 represents a schematic diagram for this type of experiment, particularly for the evaluation of HPMC-lipid edible coatings containing GRAS salts for the control of black spot on cherry tomato. Fresh fruit samples are selected, washed, artificially inoculated with the target pathogen, and, after about 24 h (curative activity), coated with the different coating treatments and allowed to dry on a mesh screen. Coatings are usually applied by fruit immersion for brief periods (10–30 s)
[29], but they can also be applied by pipetting a small amount of the emulsion (0.1–0.5 mL, depending on the commodity) onto each fruit and rubbing manually with gloved hands to mimic coating application in industrial packing line roller conveyors
[30]. Control fruits are inoculated, but are uncoated or treated with coatings formulated without GRAS salts. Depending on the experiment and the commodity, inoculated and treated fruits can be incubated at 20–25 °C or cold-stored, similar to commercial postharvest handling. Sample size, dependent variables, and statistical analyses used for this kind of
in vivo trial are equivalent to those described above for the
in vivo evaluation of GRAS salt aqueous solutions.
Figure 1. Methodological procedure for formulation and in vivo evaluation of the ability of edible coatings containing GRAS salts to control black spot of tomato caused by the fungus Alternaria alternata.
Once the coatings with the greatest ability to control disease are identified, and as a last step for the selection of the most feasible antifungal coatings for each particular application, it is very important to determine the effect of coating application on the physiological behavior and overall quality of coated fruit. For this purpose, both physicochemical and sensory fruit quality attributes are periodically evaluated during and after cold storage and simulated periods of shelf life at 20 °C
[31]. Typically, the physicochemical fruit quality attributes that are assessed include weight loss (percent loss with respect to initial weight), fruit firmness (different types of measures with texturometers or penetrometers depending on the commodity), respiration and/or internal gas concentration (O
2 and CO
2 by gas chromatography), and overmaturation volatiles (ethanol and acetaldehyde contents by gas chromatography). Sensory fruit attributes such as flavor, off-flavors, and external and internal visual appearance should be evaluated by several trained judges with expertise in each particular commodity. In some cases, consumer tests by some nontrained individuals can also be of value.
3. Noteworthy Research and Commercial Results Obtained with GRAS Salts
Our research group at the IVIA CTP has worked for many years on evaluating GRAS salts for the control of fresh fruit postharvest decay. In vitro tests for preliminary selection of GRAS salts with activity against strains of
B. cinerea and
A. alternata pathogenic to cherry tomato fruit were performed in collaboration with researchers from Brazil
[32], and against strains of
M. fructicola pathogenic to plum fruit in collaboration with researchers from Turkey (
Table 1)
[33]. Most of the group’s work, however, has focused on evaluating the effectiveness of aqueous solutions to control postharvest diseases of fresh fruit of economic importance in the Mediterranean area of the Iberian Peninsula. More recently, we started a research line devoted to developing and selecting edible coatings containing antifungal GRAS salts effective for the control of major postharvest diseases of citrus, stone fruits, tomato, persimmon, and pomegranate.
Table 1. Percentage inhibition of radial growth of Monilinia fructicola on potato dextrose agar (PDA) Petri dishes amended with different concentrations of GRAS salts after 7 days of incubation at 25 °C.
|
| GRAS Salt |
|
| Inhibition of Monilinia fructicola (%) 1 |
|
|
| Salt Concentration (%, w/v) |
|
|
| 0.2 |
|
| 1.0 |
|
| 2.0 |
|
|
| Ammonium carbonate |
|
| 100.00 iA |
|
| 100.00 eA |
|
| 100.00 cA |
|
|
| Ammonium bicarbonate |
|
| 100.00 iA |
|
| 100.00 eA |
|
| 100.00 cA |
|
|
| Potassium carbonate |
|
| 81.76 gA |
|
| 100.00 eB |
|
| 100.00 cB |
|
|
| Potassium bicarbonate |
|
| 89.22 hA |
|
| 98.01 dB |
|
| 100.00 cC |
|
|
| Potassium silicate |
|
| 11.08 bA |
|
| 100.00 eB |
|
| 100.00 cB |
|
|
| Potassium sorbate |
|
| 49.42 efA |
|
| 100.00 eB |
|
| 100.00 cB |
|
|
| Sodium carbonate |
|
| 96.37 hiA |
|
| 100.00 eB |
|
| 100.00 cB |
|
|
| Sodium bicarbonate |
|
| 100.00 iA |
|
| 100.00 eA |
|
| 100.00 cA |
|
|
| Sodium acetate |
|
| 22.64 cA |
|
| 62.21 bB |
|
| 93.98 bC |
|
|
| Sodium diacetate |
|
| 35.83 dA |
|
| 100.00 eB |
|
| 100.00 cB |
|
|
| Sodium benzoate |
|
| 42.34 deA |
|
| 91.92 cB |
|
| 99.67 cC |
|
|
| Sodium formate |
|
| 0.00 aA |
|
| 60.44 aB |
|
| 92.50 aC |
|
|
| Sodium propionate |
|
| 54.37 fA |
|
| 100.00 eB |
|
| 100.00 cB |
|
|
| Sodium methylparaben 2 |
|
| 24.85 cA |
|
| 100.00 eB |
|
| 100.00 cB |
|
|
| Sodium ethylparaben 2 |
|
| 27.80 cA |
|
| 100.00 eB |
|
| 100.00 cB |
|
Note: Means in rows with different capital letters and means in columns with different lowercase letters are significantly different by Fisher’s protected least significant difference (LSD) test (P < 0.05) applied after ANOVA. 1 Colony diameter reduction with respect to control treatments (nonamended PDA plates). 2 Doses of the agents tested were 0.01, 0.05, and 0.1%. Reproduced from Karaca et al. (2014) [33] with permission from Elsevier.