Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by Jessie Wu and Version 3 by Jessie Wu.
This entry describes entrectinib as an antiviral drug.
- SARS-CoV-2
- COVID-19
1. Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the pathogen that causes the novel coronavirus disease-2019 (COVID-19). First detected in Wuhan, China, in December 2019, it quickly spread across the country and, by 11 March 2020, the World Health Organization (WHO) declared COVID-19 a global pandemic, present in almost every country across the globe. Since then, as of November 2021, 260 million people have been infected, counting 5.2 million deaths worldwide [1].
SARS-CoV-2 belongs to the broad family of coronaviruses, a group of RNA viruses that infect mammals and birds. They are enveloped viruses with a positive-sense single-stranded RNA genome and a nucleocapsid of helical symmetry. In humans and birds, they cause respiratory tract infections that can range from mild to lethal. Particularly, SARS-CoV-2 is a member of the betacoronavirus genus. Some betacoronaviruses, such as HCoV-OC43 and HCoV-HKU1, cause mild to moderate respiratory illnesses such as the common cold (which is also caused by other viruses, predominantly rhinoviruses). However, other betacoronaviruses are responsible for severe respiratory illnesses that can pose a threat to human health, including not only SARS-CoV-2 but also severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronaviruses, which have resulted in previous epidemics [2][3][4].
Cellular infection with SARS-CoV-2 is initiated by the binding of the spike protein to its cellular receptor angiotensin-converting enzyme 2 (ACE2) [5]. This triggers the endocytosis of virions, with the envelope intact. Once inside the endosomal vesicle, induced lysosomal proteases cleave the spike protein, mediating fusion of the viral envelope with the endosomal membrane [6]. Subsequently, the viral genome is released into the cytosol, where it starts transcription and replication processes [7].
Major pharmaceutical companies have focused on vaccine development as the primary response to the COVID-19 outbreak. At the same time, numerous research groups and pharmaceutical companies are looking for antiviral drugs to be used as non-immunological interventions. Several drugs targeting viral proteins are being used or are under investigation for use against SARS-CoV-2. Some of the main targets are the spike protein, the RNA-dependent RNA polymerase (RdRp or nsp12), and the main protease (Mpro or nsp5). This is the case of remdesivir (RdRp inhibitor) [8], ritonavir, lopinavir, and PF-07321332 (inhibitors of viral proteases) [9][10][11], or plitidepsin (inhibitors of the viral replication) [12]. Particularly, antiviral drugs targeting the spike protein are considered a promising strategy, as they block the host cell recognition and viral entry. For instance, therapeutic antibodies targeting the spike protein have been approved by the Food and Drug Administration (FDA) for the treatment of COVID-19 (e.g., Bamlanivimab + Etesevimab, Casirivimab + Imdevimab) [13]. In addition, drugs that interfere with the host cell lipid metabolism, which is critical for viral infection (e.g., inhibitors of the acid sphingomyelinase activity), are of particular interest [14][15] and have shown favorable results in in vitro [16] and clinical [17] studies.
2. Targeting the RBD–ACE2 Interface for a SARS-CoV-2-Specific Antiviral Action
A promising strategy to prevent SARS-CoV-2 infections is to inhibit the initial step of viral entry into the host cell. The SARS-CoV-2 spike protein and in particular the receptor-binding domain (RBD) are crucial for viral attachment to the ACE2 host cell receptor.
2.1. Molecular Dynamics Simulations of the RBD–ACE2 Interface Reveal Contact Hotspots
To identify which residues of the RBD are most relevant for establishing the ACE2 interactions, we explored the RBD–ACE2 interface in long-scale molecular dynamics simulations with 10 μs of simulation time (Figure 1A). Relevant hotspots were detected by computing the contact frequencies between individual residues. Critical regions with more than 90% contact frequencies were mapped on the RBD–ACE2 interface and involved the following residues: ARG439, GLN493, GLY496, THR500, ASN501, GLY502, and TYR505 (Figure 1B). They represent valuable information to guide the subsequent virtual screen for drug candidates that are able to interfere with the viral attachment interface.
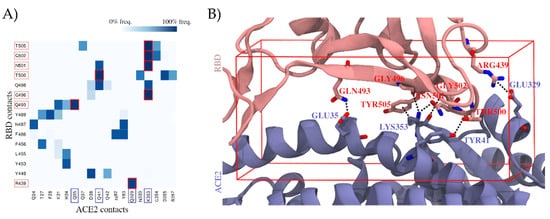
Figure 1. Contribution of individual residues to the stability of the binding complex formed by the spike protein’s receptor-binding domain (RBD) and the angiotensin-converting enzyme 2 (ACE2), computed as contact frequencies. (A) Stability of RBD–ACE2 contacts. Heatmap of contact frequencies between RBD and ACE2 residues, where contact frequencies are represented as a color scale from white (0%) to dark blue (100%). In the red square are depicted those contacts that are maintained through >90% of the simulation, and the corresponding RBD and ACE2 residues are highlighted with salmon and blue squares, respectively, in the axis labels. Residues that do not form any interaction with frequency >50% were filtered out. (B) Structural mapping of the most stable contacts (contact frequency >90%) between RBD and ACE2. The interface region where these contacts are found was used to guide a virtual screening. The docking box applied in virtual screening is indicated in red.
2.2. Virtual Screening Yields Two Drug Candidates with the Potential to Inhibit SARS-CoV-2 Cell Entry
For our drug repurposing strategy, we created a curated database of 5849 compounds including drugs approved by the FDA and the European Medicines Agency (EMA), as well as known drug metabolites. The curated database was docked into the RBD–ACE2 interface focusing in particular on regions important for the complex stability determined in the previous step (see docking box in Figure 1B). We carried out two individual screens targeting the binding interface of the RBD and the ACE2.
Among the top hits of both screenings, we manually selected five promising candidates to be tested in a cell-based assay (Table 1). In addition, we included four more compounds that had been related to antiviral SARS-CoV-2 activity. (i) Ivermectin, with a proven antiviral effect, was proposed to bind the RBD [18], as well as (ii) argatroban and (iii) otamixaban—two compounds that have been suggested to inhibit viral cell entry in a computational study by interfering with the transmembrane protease TMPRSS2a [19]. Ultimately, we included (iv) apilimod as a positive control—a PIKfyve inhibitor [20] that has been demonstrated to block the viral entrance in vitro [21] (Table 1), likely by disrupting host endocytosis by inhibiting the early endosome to the late endosome pathway [22]. However, its value as a drug candidate against COVID-19 has been questioned, as the inhibited proteases are also critical for efficient antiviral immune responses, which would be counterproductive for the treatment [23].
Table 1. Compounds selected for the in vitro inhibition activity assay based on docking or on evidence in the literature.
| Compound Name | Source | Docking Assay |
|---|---|---|
| Nilotinib | Virtual screening | RBD docking |
| Entrectinib | Virtual screening | ACE2 docking |
| Rutin | Virtual screening | ACE2 docking |
| Diosmin | Virtual screening | ACE2 docking |
| Naldemedine | Virtual screening | RBD and ACE2 docking |
| Apilimod | Literature [21] (used as positive control in our validation experiments) |
- |
| Argatroban | Literature [19] | - |
| Otamixaban | Literature [19] | - |
| Ivermectin | Literature [18] | - |
2.3. Proof of Concept
2.3.1. Pseudovirus Assay Confirms Anti-SARS-CoV-2 Activity for Entrectinib and Nilotinib
A total of nine compounds selected from the virtual screening (five) and literature search (four) (Table 1) were tested in a SARS-CoV-2 pseudovirus assay to measure inhibition of viral cell entry, as described by Walls et al. [24]. For this initial in vitro screening, we tested the inhibitory capacity of selected candidates across six different concentrations (Figure 2).
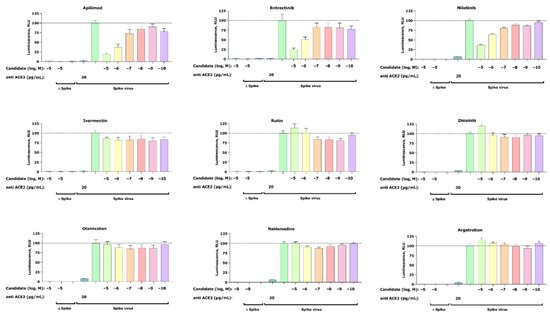
Figure 2. Inhibition of the cell entry of SARS-CoV-2 pseudotyped particles. The 293 T cells transiently expressing human ACE2 and TMPRSS2a were pre-treated with the selected compounds at the concentrations indicated for 3 h or with 20 μg/mL of the human anti-ACE2 antibody (positive control) before they were inoculated with SARS-CoV-2-specific spike protein pseudotyped lentivirus particles (spike virus) or particles without a viral envelope (Δ spike). A group of cells inoculated with spike virus or Δ spike was left untreated. At 48 h postinoculation, pseudotype virus entry was analyzed by luminescence readout (normalization against untreated spike virus entry). Data represent the mean ± SEM from three independent experiments carried out in technical triplicates. Unpaired two-tailed t-test analysis was used to calculate statistical significance. One biological experiment of Otamixaban was excluded due to technical error. Concentrations are displayed in logarithmic scale and molar concentrations. The logarithmic scale is used for better visibility of the wide range of concentrations that are used in these assays.
Our cell-based assay revealed a dose-dependent inhibitory effect of the positive control apilimod, thus confirming an appropriate experimental setup. In addition to apilimod, we found two more candidates that show dose-dependent inhibition of virus infection—entrectinib and nilotinib. Both compounds reduce viral infection at their highest concentrations of 25% (entrectinib) and 38% (nilotinib). Furthermore, no activity was found for argatroban, otamixaban, or ivermectin—compounds that had been related to anti-SARS-CoV-2 viral activity in the literature [18][19]. Finally, the effect of the isolated compounds on the cell viability was assessed with a standard MTT assay and confirmed that the observed decrease in luciferase signal is not the result of affected cell viability.
2.3.2. Apilimod, Entrectinib, and Nilotinib Inhibit VSV.G Cell Entry
To interrogate if our candidates can inhibit other enveloped viruses, we studied their inhibitory effect on the cell entry of particles pseudotyped with the membrane protein of vesicular stomatitis virus G (VSV.G). Interestingly, we found that apilimod, entrectinib, and nilotinib were able to partially inhibit VSV.G infection (Figure 3). Note that among the VSV.G active compounds (apilimod, entrectinib, and nilotinib), entrectinib showed the least VSV.G inhibitory while ivermectin, rutin, diosmin, otamixaban, naldemedine, and argatroban did not elicit any activity.
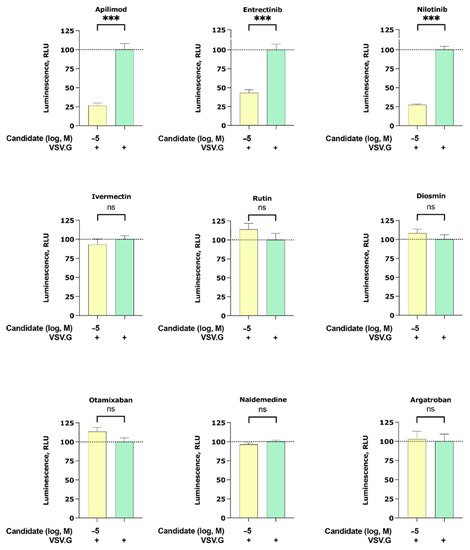
Figure 3. Inhibition of the cell entry of vesicular stomatitis virus G (VSV.G) pseudotyped particles. The 293 T cells transiently expressing human ACE2 and TMPRSS2a were pre-treated with the selected compounds at 10 µM for 3 h before they were inoculated with VSV.G envelope protein pseudotyped lentivirus particles. At 48 h postinoculation, pseudotype virus entry was analyzed by luminescence readout (normalization against untreated VSV.G virus entry). Data represent the mean ± SEM from three independent experiments carried out in technical triplicates. Unpaired two-tailed t-test analysis was used to calculate statistical significance (p > 0.05 (ns), p < 0.001 (***) compared to untreated spike virus entry). Concentrations are displayed in logarithmic scale and molar concentrations. The logarithmic scale is used for better visibility of the wide range of concentrations that are used in these assays.
2.3.4. Entrectinib Inhibits Cell Infection in Human Lung Tissue (HLT) Cells at Non-Cytotoxic Concentrations
To validate if the observed in vitro inhibitory effect of detected candidates (i.e., entrectinib and nilotinib) translates into more native conditions, we carried out an antiviral assay in HLT cells [25]. Remarkably, entrectinib exhibited a potent decrease of cellular infection without inducing significant cell death (EC50 < 1 µM) (Figure 4). Nilotinib was also found to be active but only at high concentrations (EC50 > 15 µM). Interestingly, the positive control apilimod diminished the infection rate to 50% already at very low concentrations, which points to a cytostatic effect of apilimod in this HLT assay. This effect was consistent and confirmed in two repetitive experiments (two different lung donors).
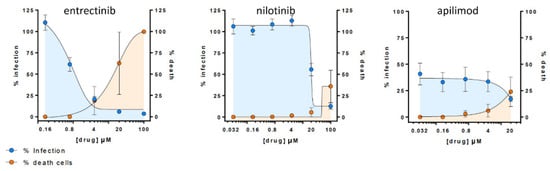
Figure 4. Antiviral assay in Human Lung Tissue (HLT) cells. Percentage of viral entry in HLT cells exposed to VSV*ΔG (Luc)-spike in the presence of the selected compounds. Non-linear fit model with variable response curve from at least two independent experiments in replicates is shown (blue lines). Cytotoxic effect on HLT cells exposed to drug concentrations in the absence of virus is also shown (orange lines).
3. Conclusion
The study reports antiviral activity of entrectinib against SARS-CoV-2 in human lung tissue, which has not been previously reported. Entrectinib is an FDA-approved drug for the treatment of solid tumors with NTRK fusion proteins and for ROS1-positive non-small cell lung cancers. Ultimately, further studies are required to confirm these data and the molecular mechanism of action for entrectinib.
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 29 November 2021).
- Chan, J.F.-W.; Kok, K.-H.; Zhu, Z.; Chu, H.; To, K.K.-W.; Yuan, S.; Yuen, K.-Y. Genomic Characterization of the 2019 Novel Human-Pathogenic Coronavirus Isolated from a Patient with Atypical Pneumonia after Visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236.
- Cheng, V.C.C.; Lau, S.K.P.; Woo, P.C.Y.; Yuen, K.Y. Severe Acute Respiratory Syndrome Coronavirus as an Agent of Emerging and Reemerging Infection. Clin. Microbiol. Rev. 2007, 20, 660–694.
- Cui, J.; Li, F.; Shi, Z.-L. Origin and Evolution of Pathogenic Coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192.
- Letko, M.; Marzi, A.; Munster, V. Functional Assessment of Cell Entry and Receptor Usage for SARS-CoV-2 and Other Lineage B Betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569.
- Zhou, T.; Tsybovsky, Y.; Gorman, J.; Rapp, M.; Cerutti, G.; Chuang, G.-Y.; Katsamba, P.S.; Sampson, J.M.; Schön, A.; Bimela, J.; et al. Cryo-EM Structures of SARS-CoV-2 Spike without and with ACE2 Reveal a PH-Dependent Switch to Mediate Endosomal Positioning of Receptor-Binding Domains. Cell Host Microbe 2020, 28, 867–879.
- Alshammary, A.F.; Al-Sulaiman, A.M. The Journey of SARS-CoV-2 in Human Hosts: A Review of Immune Responses, Immunosuppression, and Their Consequences. Virulence 2021, 12, 1771–1794.
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826.
- Lu, H. Drug Treatment Options for the 2019-New Coronavirus (2019-NCoV). Biosci. Trends 2020, 14, 69–71.
- Pavan, M.; Bolcato, G.; Bassani, D.; Sturlese, M.; Moro, S. Supervised Molecular Dynamics (SuMD) Insights into the Mechanism of Action of SARS-CoV-2 Main Protease Inhibitor PF-07321332. J. Enzyme Inhib. Med. Chem. 2021, 36, 1646–1650.
- Yavuz, S.; Komsuoğlu Çelikyurt, F.I. Antiviral Treatment of COVID-19: An Update. Turk. J. Med. Sci. 2021.
- White, K.M.; Rosales, R.; Yildiz, S.; Kehrer, T.; Miorin, L.; Moreno, E.; Jangra, S.; Uccellini, M.B.; Rathnasinghe, R.; Coughlan, L.; et al. Plitidepsin Has Potent Preclinical Efficacy against SARS-CoV-2 by Targeting the Host Protein EEF1A. Science 2021, 371, 926–931.
- Falcone, M.; Tiseo, G.; Valoriani, B.; Barbieri, C.; Occhineri, S.; Mazzetti, P.; Vatteroni, M.L.; Suardi, L.R.; Riccardi, N.; Pistello, M.; et al. Efficacy of Bamlanivimab/Etesevimab and Casirivimab/Imdevimab in Preventing Progression to Severe COVID-19 and Role of Variants of Concern. Infect. Dis. Ther. 2021, 10, 2479–2488.
- Blaess, M.; Kaiser, L.; Sommerfeld, O.; Csuk, R.; Deigner, H.-P. Drugs, Metabolites, and Lung Accumulating Small Lysosomotropic Molecules: Multiple Targeting Impedes SARS-CoV-2 Infection and Progress to COVID-19. Int. J. Mol. Sci. 2021, 22, 1797.
- Hoertel, N.; Sánchez-Rico, M.; Gulbins, E.; Kornhuber, J.; Carpinteiro, A.; Lenze, E.J.; Reiersen, A.M.; Abellán, M.; de la Muela, P.; Vernet, R.; et al. Association Between FIASMAs and Reduced Risk of Intubation or Death in Individuals Hospitalized for Severe COVID-19: An Observational Multicenter Study. Clin. Pharmacol. Ther. 2021, 110, 1498–1511.
- Carpinteiro, A.; Edwards, M.J.; Hoffmann, M.; Kochs, G.; Gripp, B.; Weigang, S.; Adams, C.; Carpinteiro, E.; Gulbins, A.; Keitsch, S.; et al. Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell Rep. Med. 2020, 1, 100142.
- Hoertel, N.; Sánchez-Rico, M.; Vernet, R.; Beeker, N.; Jannot, A.-S.; Neuraz, A.; Salamanca, E.; Paris, N.; Daniel, C.; Gramfort, A.; et al. Association between Antidepressant Use and Reduced Risk of Intubation or Death in Hospitalized Patients with COVID-19: Results from an Observational Study. Mol. Psychiatry 2021, 26, 5199–5212.
- Lehrer, S.; Rheinstein, P.H. Ivermectin Docks to the SARS-CoV-2 Spike Receptor-Binding Domain Attached to ACE2. In Vivo 2020, 34, 3023–3026.
- Rensi, S.; Altman, R.B.; Liu, T.; Lo, Y.-C.; McInnes, G.; Derry, A.; Keys, A. Homology Modeling of TMPRSS2 Yields Candidate Drugs That May Inhibit Entry of SARS-CoV-2 into Human Cells. ChemRxiv 2020.
- Gayle, S.; Landrette, S.; Beeharry, N.; Conrad, C.; Hernandez, M.; Beckett, P.; Ferguson, S.M.; Mandelkern, T.; Zheng, M.; Xu, T.; et al. Identification of Apilimod as a First-in-Class PIKfyve Kinase Inhibitor for Treatment of B-Cell Non-Hodgkin Lymphoma. Blood 2017, 129, 1768–1778.
- Riva, L.; Yuan, S.; Yin, X.; Martin-Sancho, L.; Matsunaga, N.; Pache, L.; Burgstaller-Muehlbacher, S.; De Jesus, P.D.; Teriete, P.; Hull, M.V.; et al. Discovery of SARS-CoV-2 Antiviral Drugs through Large-Scale Compound Repurposing. Nature 2020, 586, 113–119.
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of Spike Glycoprotein of SARS-CoV-2 on Virus Entry and Its Immune Cross-Reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620.
- Baranov, M.V.; Bianchi, F.; van den Bogaart, G. The PIKfyve Inhibitor Apilimod: A Double-Edged Sword against COVID-19. Cells 2021, 10, 30.
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.
- Grau-Expósito, J.; Perea, D.; Suppi, M.; Massana, N.; Vergara, A.; Soler, M.J.; Trinite, B.; Blanco, J.; García-Pérez, J.; Alcamí, J.; et al. Evaluation of SARS-CoV-2 Entry, Inflammation and New Therapeutics in Human Lung Tissue Cells. bioRxiv 2021.
More
