Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Murtaza Tambuwala.
The incidence of colon-associated diseases in the west has risen dramatically over the past several decades, as evidenced by conditions such as Crohn’s disease, irritable bowel syndrome, colorectal cancer, and ulcerative colitis.
- colon
- gut microflora
- oral drug delivery
- gastrointestinal technology
1. Introduction
The incidence of colon-associated diseases in the west has risen dramatically over the past several decades, as evidenced by conditions such as Crohn’s disease, irritable bowel syndrome, colorectal cancer, and ulcerative colitis [1]. Designing prophylactic drugs for these conditions is [2] problematic because they must cope with large pH gradients, decreased absorption, low bioavailability, and possible systemic side effects from continuous degradation [3]. In the search for novel strategies to ameliorate some of these difficulties, researchers noted that the bacterial gut microflora metabolizes host nutrients at specific locations in the colon, thereby offering a potential method of targeted drug delivery [4]. It is thought that 70% of worldwide mortality has been identified as non-communicable illnesses, including cancer, cardiovascular diseases (CVD), diabetes, chronic pulmonary disease, etc. Although traditional medicinal products (tablets, capsules, and pills) are used to treat and/or manage life-threatening and infectious illnesses, they have still demonstrated cytotoxicity, microbial resistance, and adverse response to medicines [5]. To overcome some of these unwanted consequences, research efforts have focused on efficient alternative techniques, including novel drug delivery systems, microbial delivery systems, and gene delivery systems [6,7,8][6][7][8]. Research has shown that various conditions such as cancer as well as cardiovascular and neurological disorders could be selectively targeted using microorganisms, i.e., bacteria, viruses, and fungi. Contrary to popular belief, these microorganisms are not always dangerous and can minimize negative consequences [9]. This is exemplified by the use of bacteria such as Clostridum novyi which can penetrate and inhibit the development of tumors.
The GI tract is the leading site for the action of major enzymes involved in the metabolism of food [10]. Although these enzymes can also affect the stability and availability of drugs, researchers can exploit their properties to ensure local delivery within the GI tract [11]. The intestine’s microbiome, which includes over 500 distinct bacterial species [12], is also significant for metabolism and maintaining intestinal health [13]. However, the most dominant gut microbial species that represent most of the colon flora are Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Fusobacteria [14]. Most intestine microbiotas stay in the anaerobic part of the colon, where carbohydrate fermentation is the principal source of nutrition for this microbial population [15]. This knowledge has been exploited to develop non-starch polysaccharide coatings, which undergo fermentation by colonic microbiota [16,17][16][17].
Intriguingly, the GI tract microbiome resides not only in the large intestine but is also found in the small intestine [18]. This microbial microenvironment is believed to play a vital role in the metabolic regulation in the small intestine [19]. Contrary to the large intestine, the fate of small intestine microbiota is short due to intestinal challenges such as rapid luminal flow, fluid volume, and the secretion of bacterial compounds [19]. Besides, the volume of the small intestine microbiome composition can remarkably change over a short period and is impacted by alteration in dietary intake. Although these numbers can vary considerably, the most predominant microbes found in the small intestine are genera-specific such as Clostridium, Escherichia, and Turicibacter. The Streptococcus and Veillonella species are also found in the small intestine [14].
The influence of the microbiome of the small intestine on oral drugs, formulations, and drug absorption is still unknown. Significant pharmaceutical advances have been made to improve the local targeting of drugs in the colon. However, at present, there is limited evidence of the translational efficiency of any of this research. This could be remedied by increasing evidence-based research.
2. Gut Microbiome Metabolism Specific to the Colon
Microorganisms within the intestinal tract rely mostly on undigested food for survival in the upper digestive system [20]. Most of the diet which enters the large intestines is composed of complex polysaccharides with digestive enzyme-resistant linkages. Saccharolytic bacterial fermentation usually generates helpful metabolites, whereas some bacteria use other sources of energy that generates other metabolites that are more harmful to human health [20]. Following the fermentation of carbohydrates, short fatty acids and gases are the most crucial bacterial fermentation products [21]. Generally, the gut microbiota obtains its nutrition from partially digested food. Apart from indigestible polysaccharides, the colonic microbial metabolism also offers a wide variety of complex glycans, monosaccharides, and disaccharides that are not fully absorbed on the upper GIS through excessive intake or inadequate digestion [22,23][22][23]. Many people are diagnosed with diffuse degradation inside the large intestine; except low-fermenting cellulose, undigested lignin, and complex polysaccharides are processed in the gut microbiota. Colonic organisms such as Bacteroides (resistant starch, xylan), Roseburia (resistant starch, xylan, and oligosaccharides), Ruminooccus (resistant stomach and cellulose), Bifidobacterium (oligosaccharides), Fecali-bacteria, Enterobacteria produce a synthesis of SCFAs (acetate, propionate, and butyrate) that is an important energy source [23]. Fermentation of the carbohydrates escape the proximal digestion and undergrowth oligosaccharides. SCFAs enhance phosphorylated protein kinase (AMPK) activity in the liver and muscle. AMPK is an essential enzyme that regulates cellular energy by boosting energy consumption and the beta-oxidation of fatty acids, and reduces fat and glycogen storage [23].
Additionally, the glucagon-like peptide (GLP) and ghrelin are essential in the glucose and energy balance through modulation of various intestinal hormone levels [24]. In addition, gut microbiota changes the peripheral fat storage by adjusting the epithelium expression of the quick-induced adipocyphal factor (FIAF), which acts as the circulatory lipoprotein lipase (LPL) inhibitor (FIAF), or the peroxisome proliferation active receptor-α (PPAR/γ) [25]. Acetate binds predominantly to the GPR43, GPR41, and GPR43 propionates and butyrate to the GPR41 for SCFAs. In the intestinal epithelium, the receptors GPR41 and GPR43 are expressed. SCFAs enhance the expression of PPARs, which are significant adipogenesis mediators. SCFAs boost leptin expression by adipocytes utilizing bindings with GPR41. Adipogenesis is supposed to be bound to GPR43. The resultant fatty acid composition might therefore be linked to obesity development [26,27,28][26][27][28]. Butyrate, indisputably SCFA’s most effective form of energy for human colonocytes, has a potential anti-cancer effect and can control expression levels by both inducting apoptosis in colon cancer cells and suppressing histone deacetylases [27]. Some intestinal microbes can manufacture butyrate from lactate and acetate, avoiding lactate build-up and stabilizing the intestinal environment. Propionate is a source of energy for epithelial cells and following transfer to the liver, it plays an essential part in glycogen metabolism [28]. Researchers have exploited gut bacterial metabolism to develop an array of drug delivery systems [9] based on prodrug delivery, bacterial gene therapy, polymers containing azo groups, polysaccharides containing polyol groups, and encapsulation/coating (Figure 1).
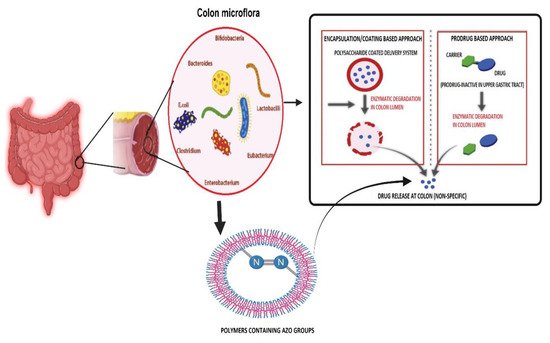
Figure 1. Illustrating various microbial metabolized approaches for colon target drug delivery systems. The pharmaceutical strategies that are commonly used to achieve a colon-specific drug delivery include time, pH-dependent polymer coating, prodrug, and the colonic microbiota azo group containing polymer-activated delivery systems, as well as a combination of these approaches. Image made by BioRender.
3. Prodrugs
Prodrugs are pharmaceutically inert parent drugs that are converted to active compounds in a specific environment [29]. In this instance, they are designed to bypass the harsh environment of upper GIT and can be activated in the colon by indigenous microbial enzymes [30]. The ideal prodrug requires effective distribution, metabolism, absorption, and elimination properties to be selective, safe, and stable toward the target site. Almost 10% of commercially available global therapeutics are considered prodrugs [31]. There are many different compounds that use this delivery route. However, one of the most popular delivery vehicles in recent years is polysaccharides. One example of this is cyclodextrin, a polysaccharide that resists hydrolytic stomach processes and is eventually broken down by cyclodextranase produced by bacteria in the gut [32]. Similar bacterial metabolism has been employed for glycoside/glycosidase-based prodrugs in colon-specific drug delivery. These have been used in carriers such as 5-aminosalicylic acid (5-ASA), which has been conjugated with metoclopramide (MCP) [23,33][23][33].
Other standard conjugates are routinely employed in drug trials, such as pectin coated in an enteric solution (Eudragit S100) to overcome poor compatibility [34] and chondroitin sulfate used to transport substances to the large bowel [35]. There are, of course, many other prodrugs such as amino acid conjugates, glycoside conjugates, glucuronide conjugates, cyclodextrin conjugates, and acetic acid conjugates [4].
4. Bacterial Gene Therapy
Specific gut microbiota, such as Clostridium, Salmonella, Bifidobacterium, Listeria, and E. coli, have been shown to accumulate and proliferate in the tumor microenvironment (TME). This allows for a delivery system known as bacterial-directed enzyme prodrug therapy (BDEPT) [36]. In BDEPT, patients are given genetically altered bacteria that express specific prodrug-activating enzymes and accumulate as well as secrete prodrug-converting enzymes within TME. When the enzyme levels are optimal, the prodrug is administered to patients and converted to an active drug specifically within TME [36].
Specific strains of E. coli DH5-lux/G have been designed to express glucuronidases that convert a sweet tasting compound usually found in liquorice root to glycrrhetnic acid in the TME. In this system, the bacteria multiply and continuously produce the therapeutic molecules that target cancer cells [37]. This BDEPT approach demonstrated that genetically engineered microbes could be an effective strategy for cancer-targeted therapy [36]. In a similar manner, recent research by a cancer group in Swansea University demonstrated that the Salmonella species could be modified to produce RNA interference, successfully reprogramming individual cancer cells to inhibit their growth.
5. Potential of the Azo Polymer-Based Hydrogel Drug Delivery System
Another important class of colon drug delivery drugs are known as azo polymers. These are dependent on the microbial reduction of the azo bond [38,39][38][39]. Most recently, this technology has been successfully incorporated into a pH-sensitive and enzyme-sensitive nanocomposite hydrogel that delivers curcumin to colon cancer cells [40]. This demonstrated good delivery kinetics and selective targeting capacity [41].
6. Encapsulation
Polymers have also been used to coat drugs used in a bacterially aided drug delivery system. Polysaccharides are a common choice for this method because of their low cost, low immunogenicity, and biocompatibility [42]. Like the concept of prodrugs, encapsulated drugs are broken down by microbial enzymes at a specific location in the digestive system. However, the polysaccharide-based drug delivery system also has some drawbacks, such as high-water solubility, but further modifications can overcome this. In a similar manner, chitosan can be used as another non-toxic, biodegradable, biocompatible, and bioactive polysaccharide. In recent experiments, it has been used to produce chitosan microcores entrapped within acrylic microspheres for the colonic delivery of sodium diclofenac [43]. These can also be coated by Eudragit, ensuring efficient pH-dependent release profiles. Similarly, other polysaccharide-based drug delivery systems are listed (Table 1).
Table 1.
A list of encapsulated/coated polysaccharide-based drug delivery systems for colon targeting.
| Polysaccharide | Delivery System | Drug Molecule | Therapeutic Application | Feature | Ref. | |
|---|---|---|---|---|---|---|
| Chitosan | Eudragit S-100 and chitosan-based nanoparticles | Paclitaxel | Colorectal cancer | Sustained-release, pH-responsive, bacterial enzyme sensitive, and cancer-targeted | [44] | |
| Dextran | The doxorubicin and superparamagnetic iron oxide nanoparticles-loaded solid lipid nanoparticle coated with folate and dextran | Doxorubicin and superparamagnetic iron oxide nanoparticles | Colon cancer | The microbial enzyme sensitive and tumor-targeted delivery system used for chemo/magnetothermal combination therapy | [45] | |
| Guar gum | The guar gum modified upconversion nanocomposite | 5-Fluorouracil | Colorectal cancer | Bacterial enzyme-sensitive and NIR-triggered | [46] | |
| Guar gum | Transformable capsules containing indomethacin immediate-release pellets | Indomethacin | Colon cancer | Bacterial enzyme-sensitive | [47] | |
| Guar gum | Microspheres | Mesalamine and symbiotic | Ulcerative colitis | Bacterial enzyme-sensitive | [48] | |
| Guar gum | 5-Fluorouracil-containing mesoporous silica nanoparticles with guar gum capping | 5-Fluorouracil | Colon cancer | Bacterial enzyme-sensitive | [49] | |
| Pectin | The pectin/modified nano-carbon sphere nanocomposite gel films | 5-Fluorouracil | Colon cancer | Bacterial enzyme-sensitive | [50] | |
| Pectin | Pectin–zinc acetate beads coated with Eudragit S100 | Pterostilbene | Colorectal cancer | pH-responsive and bacterial enzyme-sensitive | [51] | |
| Chitosan and alginate | Thiolated chitosan/alginate composite microparticulate coated by Eudragit S-100 | 5-Aminosalicylic acid and curcumin | Colitis | pH-responsive, bacterial enzyme-sensitive, and mucoadhesive | [52] | |
| Chitosan and sodium alginate | The sodium alginate-coated electrospun fiber mat containing quercetin-loaded chitosan nanoparticles and prebiotics | Quercetin and prebiotics | Colon cancer | Bacterial enzyme-sensitive | [53] | |
| Chitosan succinate and sodium alginate | Capecitabine encapsulated chitosan succinate–sodium alginate macromolecular complex beads | Capecitabine | Colon cancer | pH-responsive, bacterial enzyme-sensitive, and mucoadhesive | [54] | |
| Chitosan and alginate | Microcapsules | Interleukin-1 receptor antagonist | Inflammatory bowel disease | pH-responsive and bacterial enzyme-sensitive | [55] | |
| Chitosan and pectin | Modified citrus pectinate–chitosan nanoparticles | Cetuximab and curcumin | Colon cancer | Bacterial enzyme-sensitive, mucoadhesive, and tumor-targeted | [56] | |
| Sodium alginate and Portulaca polysaccharide | Polymeric beads encapsulating5-fluorouracil | 5-Fluorouracil | Colorectal cancer | pH-responsive and bacterial enzyme-sensitive | [57] | |
| Guar gum and pectin | Tablets coated with guar gum and Eudragit S100 | Modified apple polysaccharide and mesalamine | Ulcerative colitis | Bacterial enzyme-sensitive | [58] | |
| Hyaluronic acid and chitosan | Hyaluronic acid-coupled chitosan nanoparticles bearing oxaliplatin encapsulated in Eudragit S100-coated pellets | Oxaliplatin | Colon cancer | Bacterial enzyme-sensitive | [59,60] | [59][60] |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global cancer observatory: Cancer today. Lyon France IARC. 2018, pp. 1–6. Available online: https://gco.iarc.fr/today (accessed on 27 January 2021).
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30.
- Philip, A.; Philip, B. Colon Targeted Drug Delivery Systems: A Review on Primary and Novel Approaches. Oman Med. J. 2010, 25, 70–78.
- Kotla, N.G.; Rana, S.; Sivaraman, G.; Sunnapu, O.; Vemula, P.K.; Pandit, A.; Rochev, Y. Bio-responsive drug delivery systems in intestinal inflammation: State-of-the-art and future perspectives. Adv. Drug Deliv. Rev. 2019, 146, 248–266.
- WHO. “Noncommunicable Diseases”. Retrieved 23 September 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 21 May 2021).
- Chen, C.K.; Huang, P.K.; Law, W.C.; Chu, C.H.; Chen, N.T.; Lo, L.W. Biodegradable polymers for gene-delivery applications. Int. J. Nanomed. 2020, 15, 2131.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33.
- Pircalabioru, G.G.; Chifiriuc, M.C. Nanoparticulate drug-delivery systems for fighting microbial biofilms: From bench to bedside. Future Microbiol. 2020, 15, 679–698.
- Chang, W.W.; Lee, C.H. Salmonella as an innovative therapeutic antitumor agent. Int. J. Mol. Sci. 2014, 15, 14546–14554.
- Bissell, T.; Steele, L. Human Anatomy & Physiology. In Human Anatomy and Physiology, 8th ed.; Pearson Benjamin Cummings: San Francisco, CA, USA, 2010.
- Reinus, J.F.; Simon, D. (Eds.) Gastrointestinal Anatomy and Physiology: The Essentials; John Wiley & Sons: Chichester, UK, 2014.
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594.
- Human Microbiome Project Consortium. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214.
- Frank, D.N.; Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785.
- Sartor, R.B. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology 2010, 139, 1816–1819.
- Sinha, V.R.; Kumria, R. Polysaccharides in colon-specific drug delivery. Int. J. Pharm. 2001, 224, 19–38.
- Macfarlane, G.T.; Macfarlane, S. Fermentation in the human large intestine: Its physiologic consequences and the potential contribution of prebiotics. J. Clin. Gastroenterol. 2011, 45, S120–S127.
- El Aidy, S.; Van Den Bogert, B.; Kleerebezem, M. The small intestine microbiota, nutritional modulation and relevance for health. Curr. Opin. Biotechnol. 2015, 32, 14–20.
- Rinta-Kanto, J.M.; Sun, S.; Sharma, S.; Kiene, R.P.; Moran, M.A. Bacterial community transcription patterns during a marine phytoplankton bloom. Environ. Microbiol. 2012, 14, 228–239.
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24.
- Boyd, S.D.; Liu, Y.; Wang, C.; Martin, V.; Dunn-Walters, D.K. Human lymphocyte repertoires in ageing. Curr. Opin. Immunol. 2013, 25, 511–515.
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60.
- Zhang, T.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C. Beneficial effect of intestinal fermentation of natural polysaccharides. Nutrients 2018, 10, 1055.
- Fluitman, K.S.; De Clercq, N.C.; Keijser, B.J.; Visser, M.; Nieuwdorp, M.; IJzerman, R.G. The intestinal microbiota, energy balance, and malnutrition: Emphasis on the role of short-chain fatty acids. Expert Rev. Endocrinol. Metab. 2017, 12, 215–226.
- Conterno, L.; Fava, F.; Viola, R.; Tuohy, K.M. Obesity and the gut microbiota: Does up-regulating colonic fermentation protect against obesity and metabolic disease? Genes Nutr. 2011, 6, 241–260.
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325.
- Steliou, K.; Boosalis, M.S.; Perrine, S.P.; Sangerman, J.; Faller, D.V. Butyrate histone deacetylase inhibitors. BioRes. Open Access 2012, 1, 192–198.
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319.
- Testa, B. Prodrugs: Bridging pharmacodynamic/pharmacokinetic gaps. Curr. Opin. Chem. Biol. 2009, 13, 338–344.
- Sinha, V.R.; Kumria, R. Microbially triggered drug delivery to the colon. Eur. J. Pharm. Sci. 2003, 18, 3–18.
- Rautio, J.; Meanwell, N.A.; Di, L.; Hageman, M.J. The expanding role of prodrugs in contemporary drug design and development. Nat. Rev. Drug Discov. 2018, 17, 559–587.
- Sinha, V.R.; Kumria, R. Colonic drug delivery: Prodrug approach. Pharm. Res. 2001, 18, 557–564.
- Zou, M.J.; Cheng, G.; Okamoto, H.; Hao, X.H.; An, F.; Cui, F.D.; Danjo, K. Colon-specific drug delivery systems based on cyclodextrin prodrugs: In vivo evaluation of 5-aminosalicylic acid from its cyclodextrin conjugates. World J. Gastroenterol. 2005, 11, 7457.
- Yang, Y.; Kim, W.; Kim, D.; Jeong, S.; Yoo, J.W.; Jung, Y. A colon-specific prodrug of metoclopramide ameliorates colitis in an experimental rat model. Drug Des. Del. 2019, 13, 231.
- Ansari, M.; Sadarani, B.; Majumdar, A. Colon targeted beads loaded with pterostilbene: Formulation, optimization, characterization and in vivo evaluation. Saudi Pharm. J. 2019, 27, 71–81.
- Barros, P.D.; Dias, I.F.; Zanin, G.D.; Bunhak, É.J. Development and evaluation of dapsone tablets coated for specific colon release. Drug Dev. Ind. Pharm. 2020, 46, 246–252.
- Afkhami-Poostchi, A.; Mashreghi, M.; Iranshahi, M.; Matin, M.M. Use of a genetically engineered E. coli overexpressing β-glucuronidase accompanied by glycyrrhizic acid, a natural and anti-inflammatory agent, for directed treatment of colon carcinoma in a mouse model. Int. J. Pharm. 2020, 579, 119159.
- Available online: https://www.bbc.co.uk/news/uk-wales-south-west-wales-36712240 (accessed on 15 August 2021).
- Jain, A.; Gupta, Y.; Jain, S.K. Azo chemistry and its potential for colonic delivery. Crit. Rev. Ther. Drug Carrier. Syst. 2006, 23.
- Roldo, M.; Barbu, E.; Brown, J.F.; Laight, D.W.; Smart, J.D.; Tsibouklis, J. Azo compounds in colon-specific drug delivery. Expert Opin. Drug Deliv. 2007, 4, 547–560.
- Hou, L.; Shi, Y.; Jiang, G.; Liu, W.; Han, H.; Feng, Q.; Ren, J.; Yuan, Y.; Wang, Y.; Shi, J.; et al. Smart nanocomposite hydrogels based on azo crosslinked graphene oxide for oral colon-specific drug delivery. Nanotechnology 2016, 27, 315105.
- Ray, S. Advanced colon-specific delivery systems for treating local disorders. In Polysaccharide Carriers for Drug Delivery; Woodhead Publishing: Sawston, UK, 2019; pp. 737–762.
- Akala, E.O.; Elekwachi, O.; Chase, V.; Johnson, H.; Lazarre, M.; Scott, K. Organic redox-initiated polymerization process for the fabrication of hydrogels for colon-specific drug delivery. Drug Dev. Ind. Pharm. 2003, 29, 375–386.
- Shen, M.Y.; Liu, T.I.; Yu, T.W.; Kv, R.; Chiang, W.H.; Tsai, Y.C.; Chen, H.H.; Lin, S.C.; Chiu, H.C. Hierarchically targetable polysaccharide-coated solid lipid nanoparticles as an oral chemo/thermotherapy delivery system for local treatment of colon cancer. Biomaterials 2019, 197, 86–100.
- Kumar, B.; Murali, A.; Bharath, A.B.; Giri, S. Guar gum modified upconversion nanocomposites for colorectal cancer treatment through enzyme-responsive drug release and NIR-triggered photodynamic therapy. Nanotechnology 2019, 30, 315102.
- Yang, C.; Zhang, Y.; Cai, P.; Yuan, S.; Ma, Q.; Song, Y.; Wei, H.; Wu, Z.; Wu, Z.; Qi, X. Highly specific colon-targeted transformable capsules containing indomethacin immediate-release pellets for colon cancers therapy. J. Drug Target. 2020, 28, 102–110.
- Kaur, R.; Gulati, M.; Singh, S.K. Role of synbiotics in polysaccharide assisted colon targeted microspheres of mesalamine for the treatment of ulcerative colitis. Int. J. Biol. Macromol. 2017, 95, 438–450.
- Kumar, B.; Kulanthaivel, S.; Mondal, A.; Mishra, S.; Banerjee, B.; Bhaumik, A.; Banerjee, I.; Giri, S. Mesoporous silica nano-particle based enzyme responsive system for colon specific drug delivery through guar gum capping. Colloids Surf. B 2017, 150, 352–361.
- Wang, S.Y.; Meng, Y.J.; Li, J.; Liu, J.P.; Liu, Z.Q.; Li, D.Q. A novel and simple oral colon-specific drug delivery system based on the pectin/modified nano-carbon sphere nanocomposite gel films. Int. J. Biol. Macromol. 2020, 157, 170–176.
- Gadalla, H.H.; Mohammed, F.A.; El-Sayed, A.M.; Soliman, G.M. Colon-targeting of progesterone using hybrid polymeric microspheres improves its bioavailability and in vivo biological efficacy. Int. J. Pharm. 2020, 577, 119070.
- Wen, P.; Hu, T.G.; Li, L.; Zong, M.H.; Wu, H. A colon-specific delivery system for quercetin with enhanced cancer prevention based on co-axial electrospinning. Food Funct. 2018, 9, 5999–6009.
- Duan, H.; Lü, S.; Gao, C.; Bai, X.; Qin, H.; Wei, Y.; Wu, X.A.; Liu, M. Mucoadhesive microparticulates based on polysaccharide for target dual drug delivery of 5-aminosalicylic acid and curcumin to inflamed colon. Colloids Surf. B 2016, 145, 510–519.
- Sinha, P.; Udhumansha, U.; Rathnam, G.; Ganesh, M.; Jang, H.T. Capecitabine encapsulated chitosan succinate-sodium alginate macromolecular complex beads for colon cancer targeted delivery: In vitro evaluation. Int. J. Biol. Macromol. 2018, 117, 840–850.
- Cao, J.; Cheng, J.; Xi, S.; Qi, X.; Shen, S.; Ge, Y. Alginate/chitosan microcapsules for in-situ delivery of the protein, interleukin-1 receptor antagonist (IL-1Ra), for the treatment of dextran sulfate sodium (DSS)-induced colitis in a mouse model. Eur. J. Pharm. Biopharm. 2019, 137, 112–121.
- Sabra, R.; Billa, N.; Roberts, C.J. Cetuximab-conjugated chitosan-pectinate (modified) composite nanoparticles for targeting colon cancer. Int. J. Pharm. 2019, 572, 118775.
- Asnani, G.P.; Bahekar, J.; Kokare, C.R. Development of novel pH–responsive dual crosslinked hydrogel beads based on Portulaca oleracea polysaccharide-alginate-borax for colon specific delivery of 5-fluorouracil. J. Drug Deliv. Sci. Technol. 2018, 48, 200–208.
- Mohanta, S.; Singh, S.K.; Kumar, B.; Gulati, M.; Kumar, R.; Yadav, A.K.; Wadhwa, S.; Jyoti, J.; Som, S.; Dua, K.; et al. Efficacy of co-administration of modified apple polysaccharide and probiotics in guar gum-Eudragit S100 based mesalamine mini tablets: A novel approach in treating ulcerative colitis. Int. J. Biol. Macromol. 2019, 126, 427–435.
- Jain, A.; Jain, S.K.; Ganesh, N.; Barve, J.; Beg, A.M. Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 179–190.
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 2018, 9, 757.
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health benefits of Lactobacillus gasseri CP2305 tablets in young adults exposed to chronic stress: A randomized, double-blind, placebo-controlled study. Nutrients 2019, 11, 1859.
More
