Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Charles M. Dozois and Version 2 by Camila Xu.
Type 1 fimbriae (pili) are an important colonization factor that can contribute to diseases such as urinary tract infections and neonatal meningitis.
- Escherichia coli
- stress response
- type 1 fimbriae
1. Role of Type 1 Fimbriae in Pathogenesis
One of the most important virulence factors of pathogenic E. coli is type 1 fimbriae. This fimbrial adhesin can mediate bacterial attachment to and invasion of host cells and is subject to regulation through phase variation by a variety of environmental signals. More specifically, bacterial attachment via type 1 fimbriae to host d-mannosylated proteins will trigger signal transduction and induce actin rearrangement in target cells, allowing the pathogen to invade. In UPEC, type 1 fimbriae bind to the mannose-enriched uroplakins found on urothelial cells of the bladder [1][12]. Once internalized, the bacteria can rapidly multiply to form biofilm-like intracellular bacterial communities (IBCs) where they can evade host immune defenses and antibiotic treatments. As they proliferate, bacteria can then disperse from IBCs to colonize and invade other cells. IBC formation mediated by type 1 fimbriae is especially important for UPEC pathogenesis as it promotes bacterial ascension from the urinary tract to the kidneys [2][20]. Although the role of type 1 fimbriae has mainly been studied in UPEC, the fimbrial adhesins have also been shown to contribute to NMEC pathogenesis through adherence and invasion of human brain microvascular endothelial cells (HBMEC) [3][21]. In APEC strains, type 1 fimbriae are associated with survival, fitness, and pathogenesis by allowing more colonization of the trachea and the lung [4][5][22,23].
2. Type 1 Fimbriae
2.1. Type 1 Fimbriae Biogenesis
Fimbriae (pili) are long, proteinaceous organelles that extend from the surface of many bacteria and mediate diverse functions, including attachment, invasion, and biofilm formation. In Gram-negative bacteria, fimbriae are assembled via a range of different protein translocation systems, including the chaperone-usher (CU) pathway, the type IV secretion pathway, and the extracellular nucleation precipitation pathways [6][24].
Chaperone-usher fimbriae (CUF) are morphologically characterized as being relatively thick (~7 nm diameter), rod-like fibers with a length varying between 0.2 and 2 μm [7][25]. CU fimbriae are comprised of multiple copies (>1000) of the major fimbrial subunit and a tip adhesin that is linked by an adapter complex, which often consists of multiple minor subunit proteins [8][26]. Fimbrial subunits are shuttled through the inner membrane to the periplasm by the general secretory pathway, SecYEG translocon [9][27]. These subunits are then linked together via a zip-in zip-out mechanism coordinated by periplasmic chaperone proteins and a pore-forming usher protein, which acts as a scaffold for subunit assembly [10][28]. The chaperone facilitates several essential steps in the pathway; it mediates the folding of fimbrial subunit proteins, prevents their polymerization in the periplasm, and directs their passage to the usher. The usher in turn acts as an assembly platform and facilitates the assembly of the fimbrial structural organelle (structural component of a fimbria). Briefly, the N-terminal extension on an incoming fimbrial subunit displaces the beta-strand of the chaperone protein bound to the previously assembled subunit. Through this mechanism of strand exchange, fimbrial subunits are rapidly polymerized to form fimbriae [11][29].
Fimbrial adhesins, which are often located at the tip of the organelle, typically recognize specific receptor targets in a lock-and-key fashion, thus enabling the bacterium to target a specific surface and display tissue tropism.
2.2. Genetic Organization of Fimbrial Gene Clusters and Transcriptional Regulation
Type 1 fimbriae are among the most common adhesins in E. coli and are encoded by the fim gene cluster [12][30]. Nine genes encode the structural components and specific transport systems (fimAICDFGH), and the regulatory genes (fimB and fimE) [12][13][14][30,31,32]. FimA is the major structural subunit, which forms the majority of the extracellular filament. FimC and FimD are the chaperone and the usher, respectively, that facilitate the transport of the subunits to the bacterial surface. FimH, the adhesin tip, is integrated into the organelle structure with the help of adaptors, FimF and FimG. Although fimI is part of the operon, its function/role remains unknown; however, it is required for biogenesis of fimbriae [15][16][17][18][19][33,34,35,36,37].
The expression of type 1 fimbriae is governed by the orientation of a 314 bp invertible element (the fim switch), located immediately upstream of the major subunit gene and flanked by two 9 bp inverted repeats (5′ TTGGGGCCA) [16][34]. The expression of type 1 fimbriae is phase-variable, meaning that the promoter located within an invertible element (IE) fimS can switch between two different orientations. The phase-ON orientation (fimbriated phenotype) of the IE allows transcription of fimA and other accessory genes, resulting in the expression of type 1 fimbriae. When fimS is in the opposite orientation, no type 1 fimbrial transcription occurs, and bacteria are phase-OFF (type 1 fimbriae-negative). The inversion of the element is mediated by two site-specific recombinases, FimE, which primarily promotes switching from phase-ON to phase-OFF, and FimB, which can mediate switching in either direction [13][20][31,38].
One of the earliest phenotypic characterizations of type 1 fimbriae was their ability to confer d-mannose-sensitive hemagglutination of guinea pig erythrocytes [1][21][12,39]. Further characterization of the type 1 tip adhesin, FimH, demonstrated that type 1 fimbriae recognize mannose, which is found on the surface of many types of host cells.
3. Regulators of Stress Responses and Type 1 Fimbriae in ExPEC
The stress response can be defined as the change in gene expression of bacteria for an optimal environmental adaptation. These changes can be controlled by a specific sigma factor (e.g., master regulator, heat shock response) or another transcriptional regulator (e.g., SoxR/S or OxyR), a two-component system, the nutritional starvation response (the stringent response), or small RNAs [22][40]. These regulators can mediate changes in bacterial gene expression to adapt to stress. Some affected genes are implicated in virulence, such as type 1 fimbriae. As type 1 fimbriae play a key role in mediating E. coli host colonization and virulence, it is important to understand the regulation of these fimbriae in relation to stress responses. Indeed, numerous regulators (Figure 1) and growth conditions (Figure 2) have been identified that can affect the production of type 1 fimbriae. Below we present regulators that can play important roles in global stress regulation but have also been shown to affect expression of type 1 fimbriae (Table 1 and Table 2).
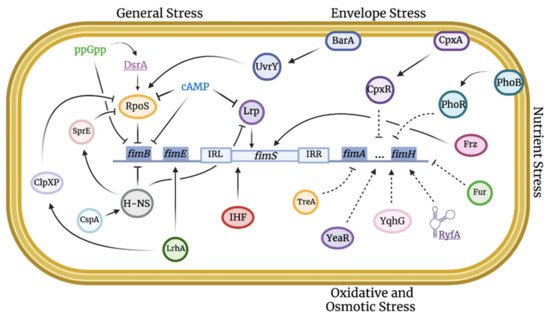
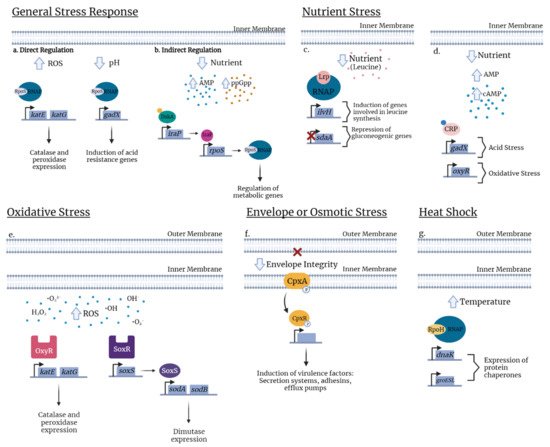

Figure 1. Integration map of stress-induced pathways implicated in type 1 fimbriae regulation. Stress regulation can be linked to virulence, such as the expression of type 1 fimbriae, through an intrinsic network of direct and indirect pathways. Solid lines indicate confirmed stimulatory or inhibitory effects. Dashed lines indicate unclear mechanisms that remain to be elucidated.

Figure 2. Examples of stress regulators in E. coli. General stress response. (a) In response to oxidative stress, RpoS occurs in direct regulation by binding to RNA polymerase (RNAP) and recognizes the promoter thus allowing expression of katG and katE catalase and peroxidase expression. Likewise, in response to low pH, binding of RpoS to RNAP induces expression of the transcriptional regulator, gadX. (b) Under nutrient limitation, RpoS is indirectly regulated by the transcription factor DskA or by the alarmone ppGpp (orange circle) that leads to the augmentation of the anti-adaptor IraP and releases RpoS to activate stress gene expression. Nutrient stress. (c) Under nutrient deficient conditions, a mis-regulation of cAMP signaling for nutrient availability allows binding of cAMP to the cAMP Receptor Protein (CRP) which activates the protein and specific binding with target DNA sequences regulating the expression of genes involved in acid stress (gadX) or in oxidative stress (oxyR). (d) In nutrient deprivation, exogenous leucine (pink circle) influences the Lrp regulon and modulates Lrp directly. Presence of leucine concentrations represses the transcription of the ilvH promoter whereas in the absence of leucine, ilvH is directly activated by Lrp. Inversely, leucine releases Lrp to bind to the sdaA promoter and activates its expression. Oxidative stress. (e) In response to oxidative stress due to excess levels of prooxidants (H2O2, O2, OH), depending on whether the stress is mediated, bacteria respond by two regulatory systems, the peroxide regulon (OxyR) or the superoxide regulon (SoxR/S). OxyR activates genes involved in catalase and peroxidase expression (katE and katG). When oxidized, the sensor SoxR activates soxS transcription resulting in expression of superoxide dismutase (sodA and sodB). Envelope stress. (f) The two-component system consists of the inner membrane, the sensor histidine kinase (CpxA) and the cytoplasmic response regulator CpxR. Envelope stress conditions lead to phosphorylation of CpxA which transfers the phosphate group to CpxR. Phosphorylated CpxR-P functions as a transcriptional regulator which controls the expression of numerous genes including some virulence factors. Heat shock. (g) In a simple pathway, during temperature upshift (30 °C to 42 °C), the Heat Shock Response (HSR) is induced by the increase of RpoH levels, primarily due to an enhanced translation of rpoH mRNA and stabilization of the protein. The elevated temperature disturbs protein homeostasis and induces accumulation of misfolded proteins. Chaperones DnaK and GroEL/S which are proteins helping to activate or degrade RpoH and regulate heat shock gene transcription.
Table 1. Global and specific stress response regulators involved in virulence and virulence gene expression in Escherichia coli.
| Regulator | Stress Response | Role in Virulence | Reference |
|---|
| RpoS |
Table 2.
Example of regulators of type 1 fimbriae in ExPEC involved in stress resistance.
| Regulator | Switch | FimE | FimB | Effect on Fim Expression | Reference |
|---|---|---|---|---|---|
| Nutrient deprivation | Master regulator of stress | [ | 23][41] | ||
| H-NS |
| General and specific stress regulators | |||||||||
| Temperature | Regulates flagellar gene expression and | fim and pap operon and many other genes | [24][42] | ||||||
| IHF | Switching on fimS | Positive or negative 1 | [36][70] | Lrp | Nutrient deprivation | Required for fim and pap fimbriae | [25][43] | ||
| ppGpp | |||||||||
| Lrp | +/− | +/− | Positive or negative 1 | [37][71] | Stringent response | Involved in biofilm formation and production of flagella | [26][27][44,45] | ||
| cAMP | |||||||||
| H-NS | − | <37 °C: − >37 °C: + |
<37 °C: Negative >37 °C: Positive |
[38][72] | Nutrient deprivation | Required for acid stress response, regulation of multiple virulence factors | [28][46 | ||
| RpoS | ] | ||||||||
| SoxS/R and OxyR | Oxidative stress | Required for virulence in UPEC | [29][47] | ||||||
| Negative | [ | 40 | ] | CpxRA | Membrane damage | Required for type 1 and P fimbriae expression in UPEC | [30][48] | ||
| [ | 73 | ] | |||||||
| ppGpp | − | Negative | [41][67] | sRNA | Diverse | MicF regulates gene expression for the outer membrane | [ | ||
| cAMP | − | Negative | [42][68] | ||||||
| Envelope stress | GadY is required for acid stress resistance | [33] | |||||||
| CpxR-P | [51] | ||||||||
| RyfA is required for survival in human macrophages, resistance to multiple stresses | [34][52] | ||||||||
| − | Negative | [ | 39 | ] | [66] | ||||
| LrhA | + | 31][49] | |||||||
| RyhB is required for nutrient stress/iron homeostasis | [32][50] | ||||||||
| Regulates the inversion | - | Negative | [ | 43][74] | |||||
| BarA/UvrY | Reduction of fimA | Unknown | Unknown 1 | [44][75] | RpoH | Heat shock | Regulates gene expression in heat shock | [35][53 | |
| Oxidative and osmotic stress | ] | ||||||||
| TreA | |||||
| Unknown | |||||
| Unknown | |||||
| Positive | |||||
| [ | |||||
| 45 | ] | [ | 76 | ] | |
| YeaR | Unknown | Unknown | Positive? 1 | [46][77] | |
| IbeA | + ? | + ? | Positive? 1 | [47][78] | |
| YqhG | Unknown | Unknown | Positive? 1 | [48][79] | |
| RyfA | Unknown | Unknown | Positive? 1 | [34][52] | |
| Nitrosative stress | |||||
| FimX | Unknown | Unknown | Positive? | [49][80] | |
| Nutrient limitation and oxygenation | |||||
| Pst and Pho regulon | + | − | Negative | [50][81] | |
| Frz | Unknown | Unknown | Positive | [51][82] | |
| Fur | Increased fimA | Unknown | Positive | [52][83] | |
| Oxygenation | Unknown | Unknown | Positive | [53][84] | |
| Biofilm and quorum sensing | |||||
| Effect of salicylate on marA | − | Negative | [54][85] | ||
| QseC/B | Unknown | Unknown | Positive | [55][86] | |
1 Putative role.
